For the majority with type 2 diabetes mellitus (T2DM), insulin therapy will be required to maintain optimal glycaemic control over time.1 The general practitioner (GP) plays a vital part in the care of patients with T2DM using insulin. This article provides a pragmatic overview of introducing insulin therapy in T2DM.
The pathophysiology of T2DM
The core pathophysiological defects leading to the development of T2DM are insulin resistance in muscle and liver cells, resulting in decreased glucose uptake and increased hepatic glucose output, coupled with failure of pancreatic beta cells to produce sufficient insulin to maintain normoglycaemia and to prevent adipose fatty acid release. This ‘glucolipotoxicity’ leads to further impairment of the beta cells, and a progressive cycle of beta cell dysfunction and metabolic decline. Although these processes are not necessarily the only mechanisms underlying beta cell decline, they remain the most potentially reversible contributors. Furthermore, it is now recognised that adipose tissue, the gastrointestinal tract, kidney and the central nervous system all play a key role in glucose homeostasis and are targets for the newer therapies for T2DM. Currently, trials are underway to examine the best strategy to preserve insulin secretion; however, until beta cell function can be meaningfully preserved, the disease is progressive.2 Most people with T2DM will require insulin to maintain optimal glycaemic control over time.
When to start insulin therapy
Initiating insulin therapy in a patient newly diagnosed with T2DM is unusual, but early insulin therapy should be considered when there is considerable weight loss, severe symptoms of hyperglycaemia or the presence of significant ketonuria. Marked ketonuria or ketoacidosis, although possible in T2DM, should prompt consideration of T1DM as the diagnosis (possibly measuring pancreatic beta cell autoantibodies and C-peptide once the patient is metabolically stable). Consultation with an endocrinologist would be prudent, particularly in the acutely unwell patient who may require inpatient or specialist ambulatory care. When T1DM has been excluded, oral hypoglycaemic agents can be trialled again in some patients, once improved glycaemic control has been established and there is some recovery of beta cell function. Here we consider the more usual and less acute situation of when and how to commence insulin therapy in patients with T2DM where glycaemic control has deteriorated despite non-insulin therapy.
Consider early: the benefits of early good glycaemic control
It is important to emphasise the benefits of early good glycaemic control in diabetes. A ‘legacy effect’ of early good glycaemic control, leading to a persisting reduction in complications and mortality, has been described.3 Furthermore, intervention when hyperglycaemia is mild can limit the weight gain often seen with insulin therapy. The message here is for practitioners to overcome therapeutic inertia and recommend intervention to treat hyperglycaemia as soon as detected.4
Establish an appropriate glycaemic target
Much depends on the individualised glycaemic target for a given patient. For example, in the young patient with T2DM, lifetime risk of complications is great and more stringent glycosylated haemoglobin (HbA1c) targets are appropriate. If already on maximal oral therapy, such patients will benefit from the early introduction of insulin, even if the HbA1c is only minimally elevated above 7%. However, in elderly patients without microvascular disease, whose prognosis is driven mainly by macrovascular risk and for whom stringent glycaemic control offers fewer benefits, a higher glycaemic threshold before insulin initiation would be appropriate. Considerations and suggested HbA1c targets are reviewed elsewhere.5,6
Check for barriers to good control
Prior to the initiation of insulin, due consideration should be given to the presence of hidden barriers to glycaemic control, such as reduced adherence to treatment for fear of hypoglycaemia, concomitant depression affecting self-care or lifestyle issues such as a penchant for sugar-rich beverages. However, it is recommended that a persistent elevation in HbA1c for 3 months or more requires more pharmacological intervention.
Insulin or a newer agent: which one should be next?
Insulin is no longer the ‘last resort’ therapy in T2DM and more recent treatment algorithms have promoted insulin as a second-line agent to metformin, depending on clinical context. A key consideration is the expected HbA1c-lowering efficacy of each agent; insulin has the greatest glucose-lowering effect. An HbA1c above 9% on maximally tolerated oral therapy should prompt consideration of insulin treatment. The reader should also consult the most recently published Australian Diabetes Society position statement on the management of glucose in T2DM.7 The GP has an important role in the intensification of therapy; if in doubt, however, it would be reasonable in the context of the newer agents and various combination options available, to seek specialist opinion with regard to the most appropriate treatment. Also, in some cases, a second supportive opinion facilitates the patient’s acceptance of insulin therapy.
Many patients when faced with the need for additional treatment will suggest a period of renewed attention to lifestyle as an alternative. We observe that the average HbA1c reduction achieved by attention to lifestyle alone at this stage, is in the order of <1% (0.5–2% in trials8). This may not be sufficient and, although encouraged, should not significantly delay therapy changes.
How to start insulin
Identify blood glucose patterns to decide which insulin to use
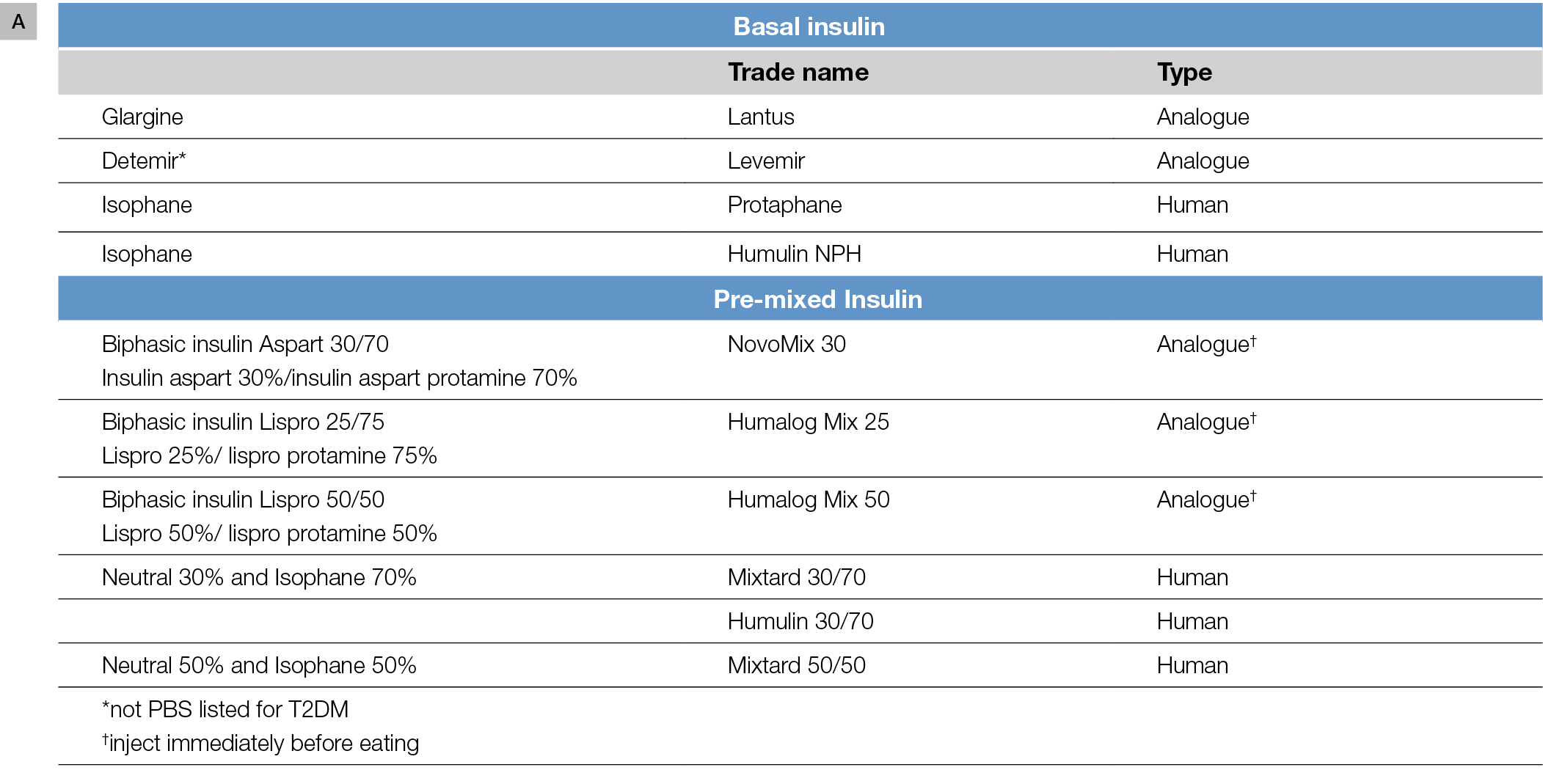
There are various options for initiating insulin therapy and, generally, initial considerations are whether to commence basal or premixed insulin. These insulin options and their action profiles are shown in Figure 1A, B.
 |
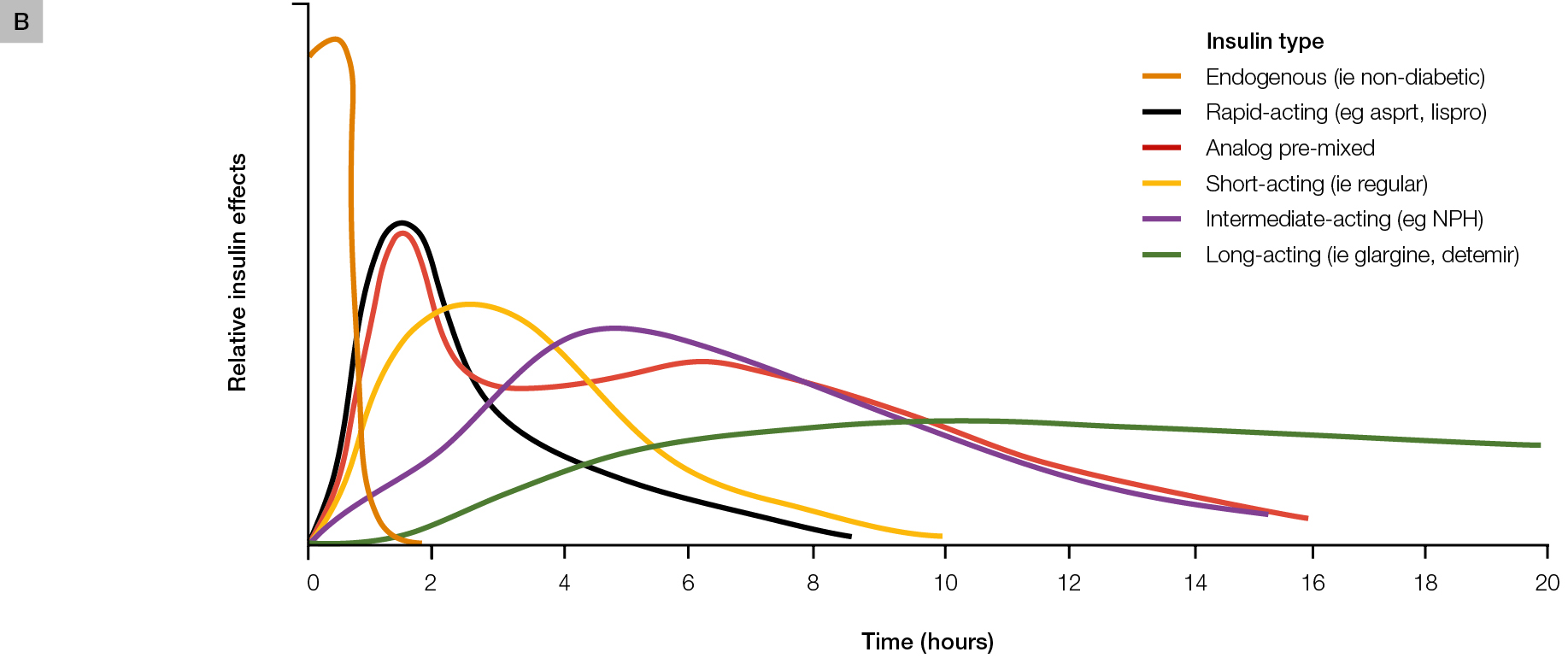 |
Figure 1. Insulin formulations
A. Insulin formulations commonly used in T2DM
B. Schematic representation of insulin time action profiles (refer to full product information for accurate pharmacokinetic profiles of each individual agent)
Panel B reproduced with permission from the American Osteopathic Association from Freeman JS. Insulin analog therapy: improving the match with physiologic insulin secretion. J Am Osteopath Assoc 2009;109:26–36. |
The first step is to identify patterns and the main periods of hyperglycaemia, with the aim of commencing with one injection of insulin a day. At this stage, self-monitoring of blood glucose is of particular benefit. The morning fasting glucose level is a useful starting point; however, our practice is to ask patients to complete a 3-day testing schedule including paired pre- and postprandial readings. General glucose targets for self testing are:
- fasting and pre-prandial: 6–8 mmol/L
- 2 hr postprandial: 6–10 mmol/L.9
 |
|
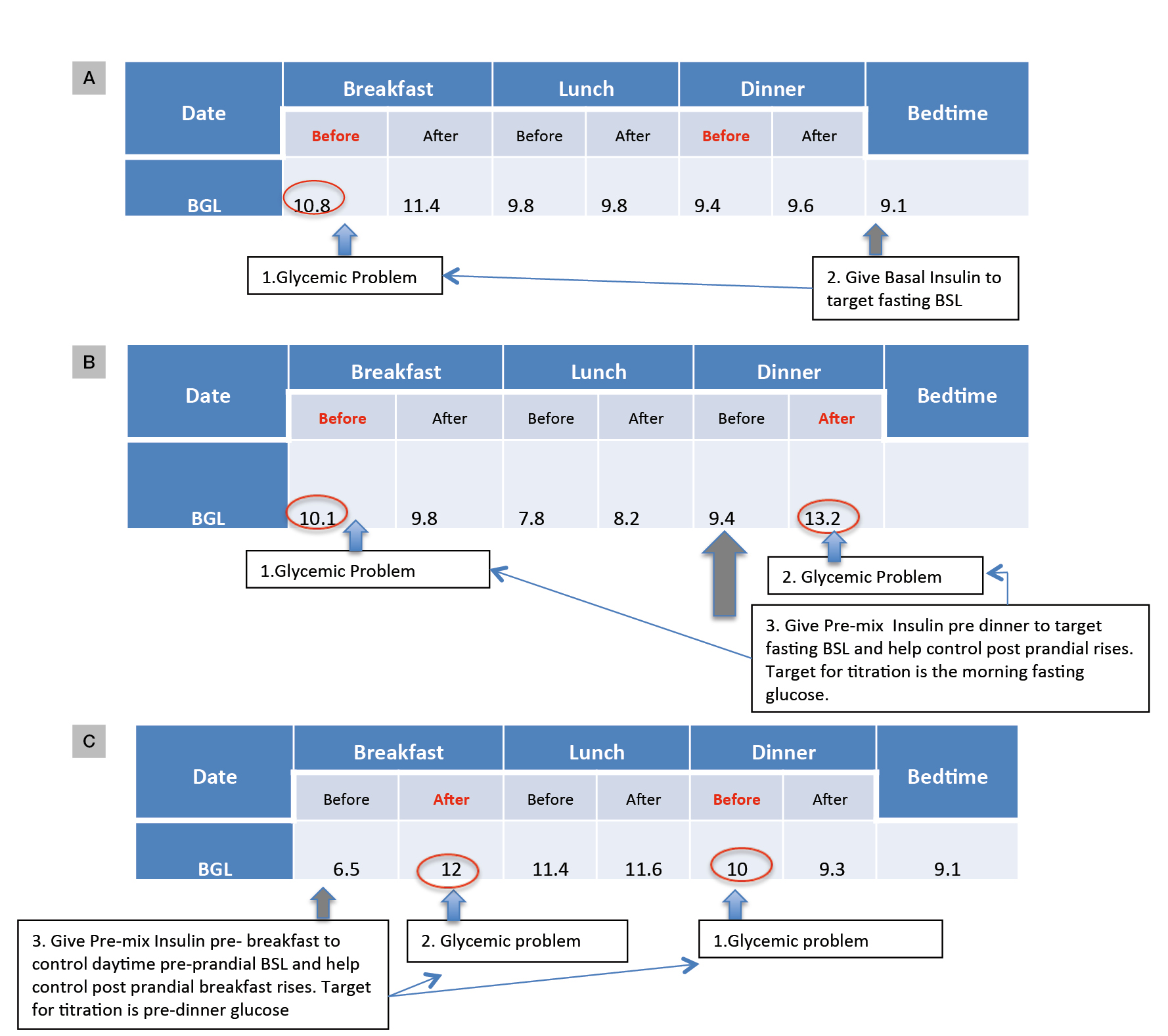
Figure 2. Examples to illustrate how SMBG patterns can guide insulin regimen choice and the timing of SMBG to guide insulin titration
A. Fasting glucose is the main problem: nocte basal insulin would target this
B. Fasting and dinnertime postprandial glucose problematic: nocte pre-mixed insulin would target both
C. Daytime hyperglycaemia with morning postprandial excursions: pre-breakfast pre-mixed insulin appropriate; suggested times for SMBG in bold red
BGL, blood glucose level; BSL, blood sugar level
|
If fasting hyperglycaemia is a problem, basal insulin would be appropriate. Pre-bed (Figure 2A) is the simplest regimen. Analogues such as glargine or detemir (not listed on the Pharmaceutical Benefits Scheme [PBS] for T2DM) are favoured, as their longer and smoother profiles are less likely to induce overnight hypoglycaemia, compared with isophane insulin.
In some patients, postprandial excursions are problematic, typically manifesting as postprandial readings >10 mmol/L with an incremental glucose rise pre-to-post prandial of about 2.5 mmol/L or greater (Figure 2B, C). Basal insulins can normalise the fasting glucose but do not affect post-prandial glucose levels, and premixed insulin formulations may be useful in patients who need to cover both fasting and post-prandial glucose. Premixed insulin analogues are appropriate here and generally preferred over human insulin, given the lower hypoglycaemia risk.10 It is important to ensure that the patient’s usual meal pattern, including the timing, frequency and carbohydrate content, are regular. If erratic meal patterns are the norm, the safest regimen to start would be basal insulin. Clinical trials comparing premixed analogues to glargine show a significant improvement in post-prandial glucose at the expense of more (but still relatively infrequent) hypoglycaemia. Preparations containing 50% rapid-acting insulin may be useful in those with high glycaemic index diets, but the 30/70 or 25/75 ratios are suitable for most and are the ideal starting choices.
Decide on a starting dose, timing and a testing schedule
The principle of ‘start low and go slow’ is prudent. The primary aims are to ensure patients can manage injecting and avoiding hypoglycaemia. It is important to emphasise to the patient that the starting dose is not likely to be the end dose and that later titration is expected.
Basal insulin can be commenced at a single low dose of 8–10 units, typically at bedtime, targeting the morning fasting glucose. Instructions should be given to test glucose in the fasting state, which is the most important for titration, and occasional checks on the pre-dinner reading.
If a pre-mixed analogue insulin is to be used once daily, a starting dose of 8–10 units is injected just prior to the meal, typically dinner. Appropriate target testing times are shown in Figure 2. In general, pre-mixed insulin is titrated to the effect of the long-acting component, with the occasional check at 2 hours post-prandial or pre-bed to reduce the risk of hypoglycaemia.
What other information to give on the first day
The main message is that administration of subcutaneous insulin injections using contemporary insulin devices is usually easily managed by the patient. This is not an ideal time for detailed dietary advice. Reinforcing the need for regular meals and snacks containing carbohydrates should suffice. The risk of hypoglycaemia is minimised by the ‘start low go slow’ approach, although the risk cannot be completely eliminated. The symptoms and treatment of hypoglycaemia should be discussed (Table 1: the rule of 1511). It is reassuring to note that in T2DM, hypoglycaemia due to insulin is usually not severe.
Table 1. The rule of 15 for management of mild
hypoglycaemia
|
|
If BGL is lower than 4.0 mmol/L, and the patient is symptomatic, alert and able to swallow safely then proceed as follows:
- Provide 15 grams of quick-acting carbohydrate that is easy to consume (eg ½ can of non-diet soft drink, ½ glass of fruit juice, 3 teaspoons of sugar, 6–7 jellybeans, 3 glucose tablets).
- Wait 15 minutes and repeat blood glucose check. If level not rising repeat step 1.
- If patient’s next meal is more than 15 minutes away, provide longer-acting carbohydrate (eg sandwich or piece of fruit). Test blood glucose again in the next 2–4 hours.
|
|
Adapted with permission from the Royal Australian College of General Practitioners. General practice management of type 2 diabetes 2014–2015. Melbourne: RACGP and Diabetes Australia, 2014.
|
What to do in the following days: titration of insulin
During the early phases of insulin treatment, a review within 7 days is prudent and a plan for titration made. Titration is to a specific target glucose level, depending on the insulin regimen chosen (Figure 2). Various titration algorithms exist.11 A suggested pragmatic algorithm, based on the lowest glucose reading over the last 3 days, is presented in Table 2. With further experience, clinicians will gain more confidence and each adjustment of dose may be larger in insulin-resistant patients, somewhere in the order of 10% of the total daily dose of insulin. Some patients may be taught to self-titrate insulin doses according to the algorithms, with regular clinical review.
Table 2. Suggested algorithm for titration of insulin dosage
|
| Lowest BGL over previous 3 days (fasting or pre-prandial)* | Adjust insulin dose once or twice weekly to achieve target BGL |
|---|
|
>10
|
↑ by 4 units
|
|
8–10
|
↑ by 2 units
|
|
7–7.9
|
No change or ↑ by 2 units
|
|
6–6.9
|
No change
|
|
4–5.9
|
↓ by 2 units
|
|
<4.0 or severe hypoglycaemic event†
|
↓ by 4 units
|
|
BGL, blood glucose level
*Adjustment should be based on the lowest BGL over the previous 3 days
†Hypoglycaemia should prompt a review of other oral therapy as well which insulin is adjusted will depend on regimen and the target glucose
Algorithm can be used for both basal and premixed insulin titration Adapted with permission from the Royal Australian College of General Practitioners. General practice management of type 2 diabetes 2014–2015. Melbourne: RACGP and Diabetes Australia, 2014.
|
When to stop other medications
It is our practice to continue metformin and other agents at the commencement of insulin, the advantage being that any addition will improve glucose levels from baseline. Should oral therapy be discontinued at this time, glucose rises are common and the patient may misinterpret this as a failure of insulin therapy. The cessation of oral agents often mandates a 20–30-unit increase in the total daily dosage of insulin, and oral agents may continue to provide an insulin-sparing effect. However, if pioglitazone is being taken, adding insulin may exacerbate oedema and, in the absence of glycaemic benefit, it could be discontinued.
Longer term, our practice is to continue at least metformin with insulin. Some endocrinologists withdraw the sulphonylurea component when pre-mixed insulin is used. The situation with the later cessation of newer agents such as dipeptidyl peptidase 4 (DPPI-4) inhibitors, sodium-glucose co-transporter 2 (SGLT-2) inhibitors and glucagon-like peptide 1 receptor agonists (GLP1RA) will be driven by the need to adhere to PBS guidelines and patient preference, although each agent can be used in combination with insulin with benefit. Studies have shown combining incretin-based therapies with basal insulin provides complementary glucose-lowering benefit. The additional weight-sparing effect of GLP1RAs may be of benefit to patients concerned about insulin-induced weight gain.12 Similarly insulin-sparing effects have been shown with the combination of DPP-4 inhibitors, leading to less hypoglycaemia.12 Nevertheless, hypoglycaemia risk is still present when combining these agents with insulin, which may prompt their cessation. Although these combinations may not be currently supported by the PBS, they are becoming more widely used in clinical practice.
When to consider more complex insulin regimens
Typically patients are on about 20–30 units of insulin in combination with oral agents when fasting or pre-prandial blood glucose levels are at target. Despite this, the HbA1c may remain elevated. In this situation, moving to a twice daily dose of insulin could be considered and specific attention paid to controlling post-prandial rises. For some, the addition of a short-acting insulin analogue before the larger meal, the so-called ‘basal plus’ regimen, will be appropriate. In T2DM, the need to provide detailed insulin-to-carbohydrate ratios is not usually necessary; however, providing a prandial insulin dose range for larger and smaller meals may be helpful.
Overcoming inertia and other clinical notes
It is our practice to introduce the idea of insulin and to administer a dummy injection of saline to demystify insulin delivery in early consultations, and to allow concerns to be explored early. At initiation, it is beneficial for the patient to have given the first dummy injection to get this out of the way before the technical aspects of the delivery device are explained. The 6 mm needle size is suitable for most. Diabetes nurse educators and registered dietitians can help greatly. It would be appropriate to review obligations related to driving licences and occupational hazards.
Conclusion
In conclusion, insulin therapy in patients with T2DM can be initiated simply and safely in the ambulatory care setting and further titration intensification can also be so managed once the patient and physician are comfortable. The appropriate choice of insulin regimen, based on patient factors and directed self-monitoring of blood glucose, together with a multidisciplinary approach can assist the successful transition to insulin.
Competing interests: Jencia Wong herself and on the behalf of the institutions to which she is affiliated have received research funds, travel grants and speaker honoraria from various companies including Eli Lilly and Company, Boehringer Ingelheim, Novo Nordisk, Merck, AstraZeneca, Bristol-Meyers Squibb, Novartis, Sanofi and Servier. Eddy Tabet has received support from MSD.
Provenance and peer review: Commissioned, externally peer reviewed.