Fixed drug eruption due to dexketoprofen
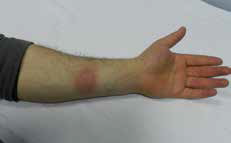
Figure 1. Erythematous-violet plaque on the flexor aspect of the left forearm |
A patient, aged 26 years, presented to our clinic after having a lesion on the ventral aspect of his left forearm repeating twice during the past month. The patient informed us that one day before the lesion erupted, he used dexketoprofen trometamol 25 mg (commercially unavailable in Australia) for his limb myalgia. The lesion subsequently resolved in about 10 days. He did not remember if he had used any other drugs besides dexketoprofen. There was no past medical history of systemic diseases such as autoimmune connective tissue disease or known skin illness in the past.
During the dermatological examination, we noticed a sharply marginatedoval, erythematous-violet plaque, 3 x 3.5 cm in diameter, on the flexor aspect of the left forearm (Figure 1). The patient declined a punch biopsy so this was not performed. The remaining physical examination and routine laboratory investigations were normal. Clinical findings were compatible with fixed drug eruption (FDE) as a result of using dexketoprofen and we treated the lesion with steroid cream (metilprednisolon aseponat, 0.1%; Figure 2). It resolved with slight hyperpigmentation in about 1 week.
Two months later, on receiving the patient’s consent, we performed an oral challenge test with dexketoprofen (6.25 mg, one-quarter of therapeutic dosage) in the hospital. The lesion recurred at the same site on his left forearm after taking this dose of dexketoprofen (Figure 3). Therefore, we concluded that the final diagnosis was FDE due to dexketoprofen in view of the history and drug challenge test. We informed the patient about the FDE and recommended avoidance of similar drugs.
|
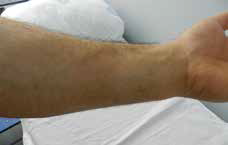
Figure 2. Slight pigmented macule on the left forearm |
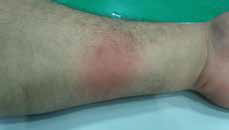 |
| Figure 3. Erythematous plaque on the flexor aspect of the left forearm (after oral challenge test with dexketoprofen) |
FDE is an uncommon cutaneous drug reaction, characterised by recurrence at the same site or sites each time when a similar drug is administered. Although the exact pathomechanism of FDE is still unclear, it is considered a delayed-type (type IV) hypersensitivity reaction. It has been reported that FDE occurs mainly in response to antibacterial and non-steroidal anti-inflammatory drugs (NSAIDs).1–2 Dexketoprofen, an NSAID that is a propionic acid derivative, is widely used for several painful disorders including musculoskeletal pain and dysmenorrhea. It has many side effects, particularly on the gastrointestinal system, but not much has been reported in the literature about its cutaneous side effect. Asensio et al3 reported a case of photocontact dermatitis (a delayed-type hypersensitivity reaction) due to oral dexketoprofen. As far as we know, there is no case of FDE due to dexketoprofen that has been reported in the literature to date. Among other NSAIDs that are propionic acid derivatives, ibuprofen and naproxen have been most frequently reported to cause FDE.1 We think that this case is worth mentioning because it is the first report of dexketoprofen-induced FDE. This case also highlights the possibility that FDE may be a side effect of different types of NSAIDs. |
Berna Kilic,
Tuba Cetiner
Teoman Erdem
Department of Dermatology, Faculty of Medicine, Sakarya University, Turkey
Erratum
Rathnayake D, Sinclair R. Tropical and exotic dermatoses and ulcers. Aust Family Physician 2014;43:604–09.
Due to a production error, Figure 5 was incorrectly labelled as ‘Single and multiple lesions of cutaneous leishmaniasis’. The figure should instead be labelled as ‘Single and multiple lesions of cutaneous larva migrans’.
Hall D, Shah, S, Ng B, et al. Changes in mood, depression and suicidal ideation after commencing pregabalin for neuropathic pain. Aust Family Physician 2014;43:705–08.
The qualifications for the second author, Simon Shah, incorrectly included BPharm. The correct qualifications are MPharm and MSc (Clinical Pharmacy).
We apologise for these errors and any confusion they may have caused our readers.