The goal of assisted reproductive technology (ART) should be the provision of safe, efficient and affordable care to optimise the chance(s) of having singleton pregnancies and the delivery of healthy babies. Recent advances in ART have focused on the achievement of this goal through biochemical and genetic research.
In the past 30 years over 3.5 million babies have been born worldwide after in vitro fertilisation (IVF) treatment. In Australia, 3.3% of all babies born in 2008 (56 923 births) were a result of IVF,1 and many more were associated with ovulation assistance or artificial insemination.
Redefining infertility and its prevalence: a contemporary approach
Historically, infertility has been defined as the failure to achieve pregnancy within 1 year of regular, unprotected intercourse.2 However, this definition does not acknowledge the importance of a woman’s age, nor does it take into consideration the requirement for regular ovulation to optimise conception chances. A more workable definition would be ‘the failure to achieve pregnancy after 12 months of unprotected and frequent intercourse in the context of regular ovulation, in a woman aged less than 37 years’. There must be recourse to earlier evaluation if a woman is 37 years or more, has risk factors for infertility (including irregular ovulation, pelvic pathology, a family history of premature menopause) or there is a risk of male infertility. In these circumstances, referral after 6 months, or even immediately, may be appropriate.
Humans are not particularly efficient at reproduction compared to many other mammals. The monthly chance of getting pregnant (fecundability) is about 25–30% for the first 3–6 months of trying. Approximately 85% of couples that will ultimately conceive spontaneously do so in the first 12 months.
Approximately 1 in 5 couples seek fertility advice from a doctor and over half of these couples require specialist assistance.3
The term ART encompasses a range of strategies to help people conceive. While IVF is the most well known treatment for significant infertility, other options include:
- ovulation induction with tablets (partial oestrogen agonists such as clomiphene) or injections (usually commercially produced follicle stimulating hormone [FSH] and luteinising hormone [LH])
- ovulation induction coupled with intrauterine insemination whereby semen is prepared in the laboratory into a highly concentrated sample for insertion through the cervix into the uterine cavity.
Fertility assistance methods are listed in Table 1.
Table 1. Fertility assistance methods
| | Ovulation induction | Ovulation induction with intrauterine insemination | In vitro fertilisation |
|---|
| Indications |
- Irregular ovulation
- Anovulation
|
- Mild infertility with normal sperm and patent tubes
- Difficulty with sexual intercourse
- Requirement for donor sperm
|
- Pelvic pathology
- Poor sperm
- Significant infertility (age, duration)
|
| Methods |
- Partial oestrogen agonists or aromatase inhibitors
- FSH (and sometimes LH) subcutaneous injections
|
- Tablets or injections as for ovulation induction
- Sperm preparation and insertion through the cervix
|
- Ovulatory stimulation with injections
- Extraction of oocytes in minor procedure
- Fertilisation of oocytes
- Embryo transfer 2–5 days later
|
| Success |
- 40–80% ovulation rates
- 15–40% pregnancy rates per cycle
|
- 10–20% pregnancy rates per cycle
|
- Pregnancy rates depend on maternal age
- 20–45% per embryo transferred
|
| Risks |
- Multiple pregnancies
- Hyperstimulation
|
- Multiple pregnancies
- Hyperstimulation
|
- Hyperstimulation
- Thrombosis
- Infection
- Genetic risks
- Multiple pregnancies if more than one embryo transferred
|
The biggest threats to fertility
Maternal age
Maternal age is the single most important factor to affect fertility, ongoing pregnancy and the success of fertility treatment.4 It is a common misconception that IVF and other fertility assistance can override the effects of maternal age. Senescence (natural ageing) of mitochondrial and nuclear DNA contributes to the degeneration of follicles and oocytes at a rapid rate after the age of 38 years. This results in failure of fertilisation and implantation and an increasing risk of miscarriage as well as an increased risk of genetic abnormalities.5,6 There is no treatment available which slows the rate of oocyte ageing, and for many women aged in their early to mid 40s, pregnancy with their own oocytes is usually not possible. These women require donor oocytes to conceive successfully.
Paternal age over 45 years is now acknowledged as having some impact on time to conception, with some evidence of a very slight increased risk of chromosomal abnormalities in children.7
Maternal obesity
The effects of obesity on fertility are unequivocal (Figure 1).8 Polycystic ovary syndrome (PCOS) is associated with hyperandrogenism, oligoovulation and insulin resistance, but obesity itself is also independently associated with reduced implantation and an increased risk of miscarriage.
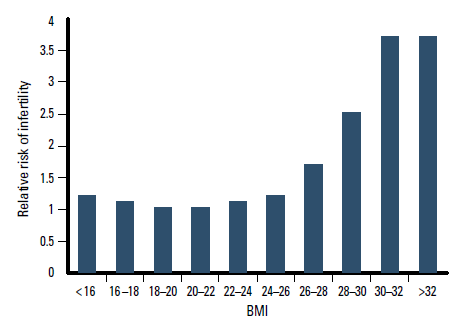
Figure 1. Relative risk of infertility by body mass index at age 18 years
Reproduced with permission from Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol 1994;171:171–7
Most useful assessments of infertility
While the causes of infertility are many, varied and often multifactorial, the tests we employ in clinical practice are relatively unsophisticated (Table 2).
Table 2. Commonly used tests to assess fertility
Ovulation
- Ultrasound surveillance of growing leading follicle
- Urine LH testing for impending surge which occurs the day before ovulation
- Serum progesterone testing, which starts to rise at ovulation and is maximal 7 days after ovulation (persists in pregnancy but starts to drop in midluteal phase if not pregnant)
Other hormone assessments as required
- Testosterone
- Sex hormone binding globulin
- Anti-Müllerian hormone
- Thyroid stimulating hormone
Pelvic assessment
- Pelvic ultrasound to visualise ovaries and uterus
- Pelvic ultrasound with fluid introduced into uterine cavity and fallopian tubes to demonstrate tubal patency (sonohysterogram and tubal patency test)
- Hysteroscopy and laparoscopy
Sperm testing
- Volume
- Concentration
- Motility (movement)
- Morphology
|
Assessment of ovarian reserve
Many women request assessment of their ovarian reserve, thinking mistakenly that there is a test that will tell them how many eggs they have left. Anti-Müllerian hormone (AMH) is produced by the granulosa cells of small growing follicles and levels diminish with age, thus reflecting the remaining pool of follicles and oocytes. It is a marker of long term ovarian function compared to early follicular phase FSH levels, which assess more immediate ovarian function. Unlike FSH, AMH levels have been demonstrated to be relatively stable throughout the menstrual cycle and also appear to be relatively independent of current hormone use (eg. the contraceptive pill).9 Anti-Müllerian hormone assessment is performed widely in infertility practice and is extremely useful in predicting ovarian response and the risk of ovarian hyperstimulation in IVF. It also has emerging usefulness in the assessment of young cancer patients after treatment.10 However, due to the enormous variability in results for women in the reproductive age group,11 it is rarely helpful, and often unnecessarily worrying, as a test of future fertility assessment (Figure 2).
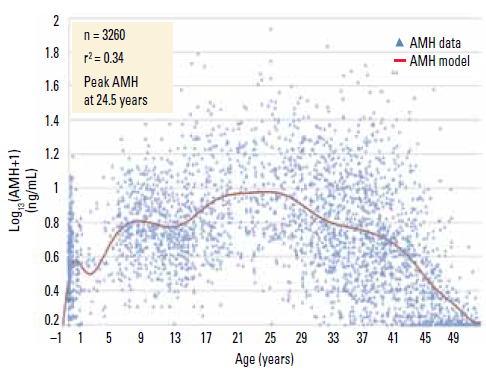
Figure 2. Anti-Müllerian hormone as a marker of ovarian reserve
Reproduced with permission from Anderson RA, Nelson SM, Wallace WHB. Measuring anti-Müllerian hormone for the assessment of ovarian reserve: When and for whom is it indicated? Maturitas 2012;71:28–33
Multiple pregnancies – the worst consequence of successful fertility treatment
Multiple pregnancies remain one of the biggest dilemmas confronting patients and fertility specialists. The risk of prematurity associated with multiple pregnancy is tenfold that with singletons, and the consequences of this can be devastating.
While there is consensus that IVF conceived twins have similar outcomes to spontaneously conceived twins, the absolute prevalence of twins has escalated over the past 20 years as a result of ART.
Ovulation induction carries a substantial risk of higher order multiple pregnancy.
Interpretation of IVF success rates
There are many ways to express pregnancy rates in ART. One of the more commonly utilised descriptors is ‘implantation rate’, which is the clinical pregnancy rate (ie. fetal heart seen) per embryo transferred. It is important when reading about success rates of ART, to be clear which denominator is being used to describe pregnancy rates, and which age range is being included, particularly when comparing different treatment strategies. Implantation rates for women aged less than 35 years approach 30–45%, but fall to less than 15% for women aged over 40 years. While rates of pregnancy using embryos generated from one stimulated cycle are about 50–60% for women aged less than 35 years, this falls to less than 20% for women aged more than 40 years.
Maximising safe and successful treatment
Lifestyle modification
Weight loss should be seen as a genuine and significant medical treatment to restore ovulation and improve fertility. A combination of exercise and dietary modification has been demonstrated to result in improved reproductive outcomes with both spontaneous and assisted fertility.12 While it is often distressing for patients and frustrating for healthcare practitioners to have to address the need for weight loss, the excellent results afforded by relatively minor weight loss – at least in the short term – should encourage women to see this as ‘do-able’.
Stress minimisation, relaxation therapy, acupuncture and naturopathy are all strategies that are commonly employed to aid fertility. It is difficult to demonstrate objective benefit, but many women and men feel more empowered by complementary therapies, at least in the early stages of infertility.
Choosing the best embryo for transfer
Great progress has been made over recent years to make pregnancy optimisation with single embryo transfer a realistic strategy. We now have genetic, morphological and metabolic tools to help us to choose the ‘best’ embryos with the highest chance of developing into healthy babies.
Genetic assessment with pre-implantation genetic diagnosis
The genetic make-up of the embryo (and the oocyte in particular) is the major factor impacting on implantation success. Chromosomal factors including aneuploidy (abnormal chromosome number) and translocations, as well as single gene defects, mitochondrial defects and defects of complex inheritance can be important. Even in young women, many embryos are found to have random aneuploidies, while embryos from older women very frequently are found to be aneuploid, and thus unlikely to progress to a normal pregnancy.13
Pre-implantation genetic diagnosis with testing of all 23 pairs of chromosomes by a microarray technique (which quantifies the amount of genetic material present of each chromosome) is a relatively new tool, which allows us to differentiate chromosomally normal from abnormal embryos and thus replace only the normal ones on day five, discarding the abnormal embryos. This improves implantation rates.14 The risk of mosaicism (different cell lines in the biopsied embryo) is 5–7%; so it is important to counsel patients that, to absolutely confirm chromosomal normality, they require an invasive prenatal assessment such as chorionic villus sampling or amniocentesis. Pre-implantation genetic diagnosis can also be utilised for translocations and single gene defects, eg. BRCA1 and BRCA2 and cystic fibrosis (Figure 3).
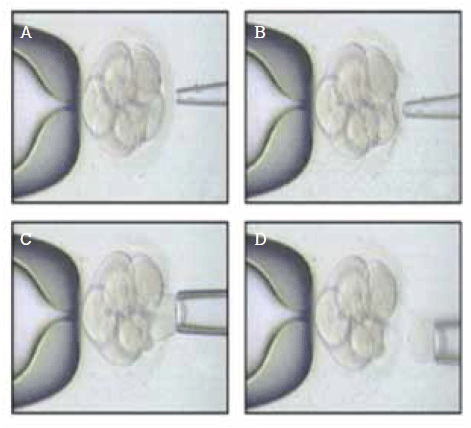
Figure 3. Embryo biopsy is performed for pre-implantation genetic diagnosis and detects aneuploidy, single gene defects, translocations and is usually indicated for recurrent miscarriage or advanced maternal age
Morphological assessment and extended culture to day five
Developments in our understanding of embryo biology have allowed scientists to identify embryos with the best pregnancy potential by observation of progress through the first few days after creation.
Only about 30–40% of embryos will continue to develop from day two to day five with considerable attrition rates. While a small percentage of the embryos which do not continue growing may have in fact survived in vivo if transferred earlier, many are destined never to implant and thus extended culture provides another means by which to help identify the better embryos from a cohort. It is important however, to appreciate that the process of extended culture does not of itself ‘make an embryo better’.
Metabolic assessment of embryo competence
Another promising tool is the measurement of glucose uptake by embryos, but this has not yet reached a stage where it can routinely be used to assess embryo competence.
Single embryo transfer
Multiple birth rates with IVF are now dropping as a result of increasing utilisation of single-embryo transfer, particularly in Australia and Scandinavia. It is interesting that the uptake of single embryo transfer is greater in countries that offer at least partially subsidised fertility treatment.15
Fertility preservation for female cancer patients
Cancer is not uncommon in young people, with approximately 1 in 570 young girls and women being diagnosed with cancer before the age of 35 years. For many young cancer patients, treatment offers a very real chance of cure and being able to have children in the future is a genuine and appropriate expectation. Many cancer treatments, especially chemotherapy and pelvic radiation, can cause ovarian failure (Table 3).16 This ovarian failure is often temporary with spontaneous resumption of menses, but it can be permanent, especially in women in the later reproductive years. Even when menstruation and ovarian function returns after chemotherapy is completed, there is still a very real risk of subsequent early onset ovarian failure.
Various options are available to young women for fertility preservation before commencement of chemotherapy or radiotherapy and include oocyte freezing, embryo freezing and ovarian tissue excision and freezing. In addition, it may be possible to protect the ovary against the toxic effects of the chemotherapy by co-administration of a long acting gonadotrophin releasing hormone (GnRH) agonist.17
It is of absolute importance that young women and their families are offered the opportunity for full discussion about future fertility, the risks and options, even if it is not going to be feasible to perform any sparing treatment.
Excellent survival can now be expected using new freeze-thawing techniques after ovarian stimulation, with an average of 10 oocytes resulting in two or three embryos being available later on. Embryo freezing is obviously most useful for young couples in long term relationships. Ovarian tissue freezing provides a potentially huge supply of oocytes in the cortical strips, but only 17 babies have been born to date after ovarian tissue grafting and this must still be considered a somewhat experimental treatment option.18
Table 3. Representative rates of ovarian failure after treatment of common childhood and young adult cancers
| Disease | Likelihood of premature ovarian failure |
|---|
| Breast cancer |
|
- Age <30 years
- Age 30–40 years
|
<10%
20–40% |
| Sarcoma |
<10–20% |
| Hodgkin disease |
<10% unless intensive therapy |
| Non-Hodgkin disease |
10–40% |
| Leukaemia |
<15% |
| High dose therapy and stem cell transplantation |
>70% |
| Reproduced with permission from Stern CJ, Seymour JF. Defining the cost of cure: infertility among female survivors of lymphoma. Leuk Lymphoma 2006;47:574–5 |
Male fertility preservation
Sperm freezing should always be considered for adolescent males and young men about to commence chemotherapy or testicular surgery. If producing a sample is difficult, then testicular biopsy under local or light general anaesthetic can be considered. There is currently much research into ways to mature sperm in vitro from very immature forms, and progress in this area will help us to provide preservation options to very young boys as well as adolescents.
Different solutions to different problems
Gamete donation
For women with extremely poor quality oocytes or premature ovarian failure, donation of oocytes offers the opportunity of a very real chance of success. Similarly, for men with azoospermia and no possibility of sperm to be obtained by testicular biopsy, donor sperm is available.
Surrogacy
Women with no uterus or a markedly abnormal uterus, or who are at risk of serious medical sequelae if they carry a pregnancy, are able to create a family using a gestational carrier (surrogate). Legislative requirements differ in different states in Australia but altruistic surrogacy is possible if women can find their own carrier.
Fertility assistance for women in same-sex relationships and for single women
It is now possible for lesbian women and single women to utilise donor sperm to assist them to create their own family.
Social fertility preservation
Many women in their late reproductive years without a partner may not wish to take the giant step of single parenthood but instead wish to preserve some oocytes for the future. As survival of oocytes after freeze-thawing improves, this becomes more of an option, but it must be remembered that there is a high rate of attrition from oocyte to usable embryo. Also, there are often a relatively low number of oocytes obtained in women in their mid to late thirties, so it is extremely important that women do not inappropriately rely on oocyte freezing as a failsafe form of ‘reproductive insurance’.
Conclusion
Over the past few years there have been numerous scientific developments that have advanced our understanding of infertility and provided us with the tools to improve both the success of infertility treatments and their safety. We are, however, still limited by our inability to combat poor (and usually age related) oocyte quality. Until we can overcome this hurdle, we will need to rely on early and appropriate recognition of infertility and prompt treatment, especially for women aged in their late 30s and early 40s. Greater insight into oocyte and embryo biology will drive further improvements in fertility treatments and perhaps, ultimately, the prevention of infertility.
Key points
- While infertility rates have not particularly increased in recent years, more couples are seeking medical assessment earlier.
- Maternal age remains the most important factor to affect fertility, pregnancy outcomes and success of fertility treatments.
- Attention to lifestyle factors, particularly maternal obesity, is important in maximising good outcomes from ART.
- While techniques for fertility preservation in the form of oocyte freezing have progressed, this should not be considered ‘fail safe’.
Conflict of interest: Melbourne IVF fertility preservation program is supplied by grants from Merck Serono, Merck Sharp & Dohme and Astra-Zeneca. The author has received payment and expenses from pharmaceutical sponsors for attending meetings.