Levels necessary for bone health
The threshold for the level of vitamin D to optimise a range of health outcomes remains controversial and randomised controlled trial evidence to support aiming for high levels is very limited. For bone health and muscle function, the optimal level appears to be ≥50 or 60 nmol/L, using the higher value if measured in summer to allow for seasonal decrease.1 A cut-off of 50 nmol/L remains appropriate to define deficiency,1,2 with further classification into severe (<12.5 nmol/L), moderate (12.5–29 nmol/L) and mild (30–50 nmol/L) deficiency.1
Seasonal variability
At a population level, 25(OH)D levels can be described with a sinusoidal curve. There is substantial seasonal variability, with 25(OH)D levels at the end of summer being around 20–35 nmol/L higher than at the end of winter.3 There are as yet no data of which the authors are aware that relate the duration of deficiency, that is the proportion of time in a given year spent under a threshold of 50 nmol/L, and health outcomes. Nonetheless, it is critical to take into account the time of year that a patient's 25(OH)D level is measured when interpreting vitamin D levels and making management choices. For example, a patient who is mildly vitamin D deficient at the end of summer will most likely be deficient all year round, and the deficiency will become more severe during winter. Therefore vitamin D supplementation might be needed. In contrast, for someone who is mildly deficient at the end of winter, a suggestion to slightly increase sun exposure at appropriate times of the day might be sufficient.
Vitamin D and muscle function
Muscle weakness can be a sign of vitamin D deficiency.4 The mechanism of this is unclear. It has been postulated that effects are mediated through actions of 1,25(OH)2D on vitamin D-specific nuclear receptor in muscle tissue,5 but the presence of such receptors has recently been questioned.6 Regardless of mechanism, poor muscle function from vitamin D deficiency may impact further on musculoskeletal health by predisposing to higher falls risk with resulting fracture (See later section). There is evidence in older adults that vitamin D affects factors directly related to muscle strength and function.5 This includes prospective data demonstrating that elderly men and women with baseline serum 25(OH)D levels <25 nmol/L, were 2.57 times more likely to lose >40% of their grip strength and over 3% of their muscle mass over a 3 year period.7 A recent meta-analysis of 17 randomised controlled trials (RCTs) involving 5072 adults demonstrated improvements in muscle strength only with vitamin D supplementation in studies in which the mean baseline 25(OH)D level of participants was <25 nmol/L (four studies, 465 participants),8 suggesting that most benefit is obtained in people with moderate to severe deficiency.
Vitamin D related osteomalacia
Osteomalacia is a bone disorder in adults in which osteoid, the newly formed bone matrix, does not mineralise. Clinical symptoms include bone pain, muscular weakness (particularly proximal muscle weakness) and difficultly with walking.9 Fractures may occur due to bone fragility. Radiological changes also include pseudofractures (Looser's zone, Figure 1), which appear as a radiolucent line through bone cortex often with marginal sclerosis. Vitamin D deficiency is one cause of osteomalacia but vitamin D levels usually need to be very low (<25 nmol/L, often <12.5 nmol/L),9 to cause clinical osteomalacia. Other causes of osteomalacia include very low calcium intake and hyposphataemia from a range of causes. Furthermore, older adults consuming inadequate dietary calcium with low serum vitamin D levels can develop both osteoporosis and osteomalacia,2 although the precise prevalence of osteomalacia in patients with hip fracture is unclear due to substantial differences in how osteomalacia is histologically defined in different studies.10
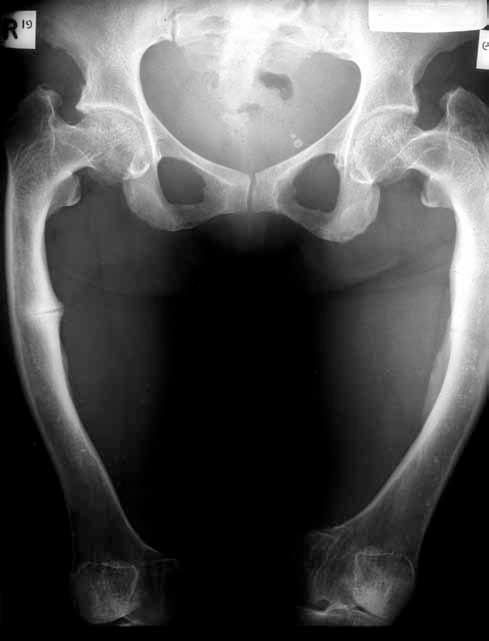
Figure 1. Looser's zone (pseudofracture) in osteomalacia. There is a radiolucent line through the medial right femoral cortex with some sclerosis at its margins
Osteoporosis, falls and fracture
Key messages
For maximal primary fracture prevention in the elderly, adequate vitamin D levels and dietary calcium intake are both needed.
The benefits of supplementation are likely to be greater in those with vitamin D deficiency and/or low dietary calcium intakes.
Low bone mineral density and falls are both risk factors for osteoporotic fractures.11 Serum vitamin D has been shown to be associated with both these risk factors in observational studies. In cross-sectional studies, serum vitamin D in the range 30–90 nmol/L has been positively correlated with hip bone mineral density in the elderly,12 but no clear threshold value has been established beyond which the relationship disappears. Most RCTs with bone density outcomes have given calcium and vitamin D concurrently, and overall effects on bone mineral density have predominantly been modest and mostly occurred in the first year of treatment. Low serum vitamin D has also been associated with falls in observational studies.13,14 Importantly, this association has been confirmed by RCT data. A meta-analysis of RCTs of vitamin D supplementation in the elderly (whether community dwelling or in institutions) demonstrated a reduction in falls risk of 19% with doses of vitamin D of 700–1000 IU/day (pooled relative risk [RR] 0.81; 95% CI: 0.71–0.92 from seven studies, n=1921) with no effects at lower doses (two studies, n=505).15 In addition, a subgroup analysis showed a 23% risk reduction (pooled RR 0.77; 95% CI: 0.65–0.90) if serum 25(OH)D levels above 60 nmol/L were achieved, but no reduction in risk if achieved levels were <60 nmol/L. Consistent with this, a meta-analysis of studies performed only in community dwelling older people demonstrated a reduction in falls-risk only in studies whose participants were selected on the basis of low serum vitamin D16 and a separate meta-analysis of studies in older people in nursing care facilities or hospitals showed a reduction in rate of falls.17 One more recent RCT has raised concerns regarding the safety of intermittent mega doses of vitamin D in the elderly, as such a regimen increased falls and fracture risk18 (See adverse affects of vitamin D).
Given that vitamin D may exert effects through improved muscle function and falls risk reduction and bone density effects, substantial weight in clinical decision making needs to be given to studies with fracture outcomes as endpoints, so as to encompass the possible benefits or detriments of the full range of vitamin D effects. There is also potential for an interplay between calcium intake and vitamin D with very low levels of calcium intake causing secondary hyperparathyroidism with increased 1,25(OH)2D which in turn may cause increased breakdown of 25(OH)D.10
| Table 1. Specific molecules often included under the generic term 'vitamin D' |
| Vitamin D3 (cholecalciferol) |
Formed in skin from UV exposure and main form found in vitamin D supplements |
| Vitamin D2 (ergocalciferol) |
Produced by UV irradiation of the plant steroid ergosterol. Found in some supplements |
| 25-hydroxyvitamin D [25(OH)D] |
Produced in liver from vitamin D2 and D3 Serum level used to measure vitamin D status |
| 1,25 dihydroxyvitamin D [1,25-(OH)2D] (calcitriol) |
Biologically active form of vitamin D, produced by hydroxylation of 25(OH)D by kidneys |
Several systematic reviews have addressed the interplay of calcium and vitamin D on fracture outcomes (Table 2). Tang et al19 reviewed trials of calcium or calcium in combination with vitamin D. In 17 trials with over 52 000 participants, in trials giving calcium alone there was no statistically significant decrease in fracture risk but in trials of calcium and vitamin D given in combination there was a statistically significant 13% risk reduction. Furthermore, in the subgroup of studies where vitamin D was co-administered, a dose in excess of 800 IU/day was needed to produce an effect (risk reduction of 16%). Greater effects were seen studies with a low mean baseline serum vitamin D (Table 1), although the p-value of 0.06 for the difference in effects was not statistically significant. No difference in effect size was seen if a cut-off of 50 nmol/L was used. This suggests that benefits were more pronounced with more severe levels of vitamin D deficiency. Unsurprisingly, in studies in which compliance with treatment was high (>80%), the risk reduction was more marked at 24%.18 Other meta-analyses have compared the effects of vitamin D alone compared to vitamin D given with calcium on fracture risk20–22 and consistently demonstrated that for there to be a reduction in fracture risk, it is necessary that vitamin D and calcium are administered together and that vitamin D alone is insufficient to reduce fracture risk. Again, the potential for intermittent mega-dose vitamin D supplementation to result in increased falls and fracture should be noted.18
| Table 2. Fracture risk reduction from meta-analyses of RCTs of vitamin D alone and of vitamin D combined with calcium* |
| All fractures |
Relative risk |
| Tang, 200719 |
|
| Calcium alone (n=6517) |
RR 0.90 (0.80–1.00)a |
| Calcium and vitamin D (n=46 108) |
RR 0.87 (0.77–0.97) |
| Vitamin D <800 IU/day (n=36 671) |
RR 0.87 (0.71–1.05)b |
| Vitamin D ≥800 IU/day (n=9437) |
RR 0.84 (0.75–0.94) |
| Serum 25(OH)D <25 nmol/L (n=10 144) |
RR 0.86 (0.78–0.93)c |
| Serum 25(OH)D ≥25 nmol/L (n=39 167) |
RR 0.94 (0.90–0.99) |
| DIPART, 201020 (n=68517) |
|
| Vitamin D alone |
HR 1.01 (0.92–1.12) |
| Vitamin D with calcium |
HR 0.92 (0.86–0.99) |
| Hip fracture |
| Boonen, 200722 |
|
| Vitamin D alone (n=9083) |
RR 1.10 (0.89–1.36)d |
| Vitamin D with calcium (n=45 509) |
RR 0.82 (0.71–0.94) |
| Avenell, 200521 |
|
| Vitamin D alone (n=18 668) |
RR 1.17 (0.98–1.41) |
| Vitamin D with calcium (n=10 376) |
RR 0.81 (0.68–0.96) |
* Bold denotes statistically significant, 95% CI does not include 1.00; RR = relative risk; HR = hazard ratio
a. No statistically significant difference in effect between the two groups, but a statistically significant effect was only seen in with calcium and vitamin D combined
b. p=0.03 for difference between <800 IU and ≥800 IU studies
c. p=0.06 for difference between <25 nmol/L and ≥25 nmol/L groups
d. Indirect comparison gives an RR reduction of 25% for vitamin D with calcium vs vitamin D alone (p=0.021) |
The role of vitamin D and calcium in secondary fracture prevention
Key messages
Vitamin D and calcium supplementation alone are inadequate for secondary fracture prevention. But optimal vitamin D levels and adequate calcium intake are needed to maximise effectiveness of anti-resorptive therapy for preventing further fractures.
The studies in the meta-analysis discussed were for primary fracture prevention, ie. prevention of the first osteoporotic fracture, with the exception of the RECORD study.23 This four-arm, secondary prevention trial compared vitamin D3 (800 IU/day), 1000 mg calcium, vitamin D3 (800 IU/day) with calcium (1000 mg/day) and placebo in 5292 mobile people (85% female) aged >70 years who had already sustained a low-trauma fracture. The incidence of new, low-trauma fractures did not differ significantly between groups. Compliance with treatment was low in this study (<50% people took tablets on >80% of days), but even in those participants with >80% compliance, no effects were seen.
RECORD demonstrates that calcium and vitamin D are insufficient by themselves for secondary fracture prevention. However, calcium and vitamin D still have an important role in secondary fracture prevention as an adjunct to specific anti-osteoporosis therapies. Most such therapies (eg. bisphosphonates) have been evaluated in the context of adequate vitamin D levels and calcium intake. In a retrospective observational study in 1515 postmenopausal on anti-resorptive therapies (alendronate, risedronate and raloxifene), the women who were vitamin D deficient (serum 25(OH)D ≤50 nmol/L) were more likely to sustain a fracture that those who were vitamin D replete (adjusted odds ratio 1.77; 95% CI: 1.20–2.59; p=0.004). Therefore optimal vitamin D levels seem necessary to maximise the anti-fracture efficacy of anti-resorptive agents.24 No studies have similarly investigated the impact of calcium repletion on fracture outcomes. However, calcium supplementation, in addition to vitamin D and alendronate in healthy postmenopausal women with low BMD with a dietary calcium intake of ≥800 mg/day did not result in any added benefit for BMD,25 indicating that providing additional calcium to this population is not necessary. Significant questions have been raised about the safety of calcium supplements due to possible increases in cardiovascular events.26 Therefore, for secondary prevention it is suggested that:
- serum 25(OH)D and dietary calcium intake are checked before initiating anti-osteoporosis therapy
- that calcium supplements are given if an adequate dietary calcium intake cannot be attained, and
- that vitamin D supplementation is essential if vitamin D levels are inadequate.11
Adverse effects of vitamin D: caution required with mega-dose therapy
Until recently, the adverse effects of vitamin D were predominantly considered to be those of relatively acute toxicity with associated hypercalcaemia; namely nausea, vomiting, constipation, anorexia, apathy, headache, thirst, sweating and polyuria. There is also a risk of renal and cardiovascular damage through ectopic calcification, especially in the presence of hyperphosphataemia.27 However, such effects are typically seen only at very high doses of vitamin D – suggested to be in excess of 25 000 IU/day with corresponding 25(OH)D levels of about 500 nmol/L2 and are highly unlikely with normal therapeutic doses.
More recently, other safety concerns have emerged with less extreme levels of serum 25(OH)D/vitamin D doses. Notably, mega-dose intermittent oral vitamin D3 (500 000 IU once per year in autumn) has been associated with a higher risk of both falls (incidence RR 1.15; 1.02–1.30; p=0.03) and fractures (incidence RR 1.26; 95% CI: 1.00–1.59; p=0.047).18 This was reported in a double-blind, placebo-controlled RCT of a single annual dose of 500 000 IU of vitamin D3 administered orally each autumn to winter for 3–5 years in 2256 community-dwelling older women aged 70 years or older and considered to be at high risk of fracture. For both falls and fracture, the greatest risk was seen in the first 3 months after dosing. This coincided with the substantial increase in serum vitamin D levels seen after each dose. Serum 25(OH)D was measured in a subsample of 137 participants. The median serum 25(OH)D rose from 49 nmol/L to approximately 120 nmol/L 1 month after taking the vitamin D supplement and at 3 months serum 25(OH)D remained high (approximately 90 nmol/L). Moreover, a quadratic relationship was seen between changes in hip flexion strength and the percent change in serum 25(OH)D. At vitamin D increases of less than 100%, hip strength increased but with vitamin D increases above this level (equivalent to 120 nmol/L), hip flexion strength decreased, providing a potential mechanism for the increased risk in falls and fractures.
In general, vitamin D3 supplements in RCTs (predominantly in elderly women in institutions and dependent care) are associated with lower mortality.28 However, of concern is the indication of a U-shaped relationship for serum vitamin D with mortality. A 50% higher total mortality rate has been observed in older men living in The Netherlands, in the lowest 10% of the distribution of plasma 25(OH)D (<46 nmol/L) as well as the highest 5% (>98 nmol/L) of the distribution.29
Preventing vitamin D deficiency: population advice
For most adults in Australia, dietary sources provide only a small (5–10%) part of their vitamin D requirement. The main source of vitamin D is exposure of the skin to sunlight (UV light). Advice to promote some sun exposure to allow the skin to manufacture vitamin D3 is important to maintain adequate serum 25(OH)D levels in the general community. This advice may be particularly important to individuals with known low levels of sun exposure who may need to make a particular effort to achieve some exposure to sunlight (eg. indoor workers).
The minimal erythemal dose (MED) is the amount of UV exposure that just causes faint erythema. This varies with latitude, season, time of day, clothing and skin pigmentation. The current vitamin D and health in adults in Australia and New Zealand position statement1 provides a guide for sun exposure times (minutes) which result in one-third MED for people with moderately fair skin at times of day that are useful for making vitamin D in different regions. If people expose around 15% of body surface (arms and hands or legs) for this amount of time on most days, this should be equivalent to around 1000 IU/day of vitamin D3. However, importantly, sun exposure advice has to be tempered with the need to avoid excessive sun exposure associated with increased skin cancer risk. Current Cancer Council recommendations are that most Australians need sun protection to prevent skin cancer when the UV index is 3 or above.30
Sun exposure guidelines for the general population
Suggested sun exposure guidelines for moderately fair-skinned individuals who are not otherwise at increased risk of skin cancer are given in Table 3. In summer, vitamin D levels are likely to be exposing the hands, face and arms for 6–7 minutes mid-morning or mid-afternoon on most days. In winter, advice is more complex, depending on latitude. In the far north of Australia, the UV index remains moderate to high throughout the year, remaining as high as 7–8 in June in Darwin for example,31 and unprotected sun exposure around noon should be avoided year round. In winter in Brisbane, sun exposure at times further from noon may result in a lower production of vitamin D. However, while the UV index around noon may be above 3,31 the gap between the time needed to gain one- third MED (11 minutes) and the time for clearly hazardous levels of sun exposure (1 MED) (34 minutes) is higher in winter.32 Therefore for most people, the short length of exposure required at noon for adequate vitamin D production (as given in Table 3) will be low risk. In winter in southern Australia, sun exposure at midday for 15–29 minutes (depending on latitude) with as much bare skin exposed as feasible, on most days, is likely to be helpful.1 Exposing more skin in winter will increase vitamin D production.33
| Table 3. Sun exposure guidelines – Australia |
| Season and location |
Advice |
| Summer (all latitudes) |
Hands, face and arms exposed for 6–7 minutes mid-morning or mid-afternoon on most days |
| Winter |
| Northern Australia, eg. Darwin, Cairns, Townsville |
Hands, face and arms exposed for 9–13 minutes mid-morning or mid-afternoon on most days35 |
| Central Australia, eg. Brisbane, Perth |
Hands, face and arms exposed for 11 (Brisbane) to 15 (Perth) minutes around noon on most days |
| Southern Australia – Sydney to Hobart |
Exposure of as much bare skin as feasible around noon most days. Duration dependent on latitude – from 16 (Sydney) to 29 minutes (Hobart) |
While one would expect broad spectrum sunscreens to reduce vitamin D production by blocking UV radiation, this may not prove to be the case in practice,34 possibly due to inadequate application. To maximise vitamin D production for the limited periods of sun exposure given in Table 3, use of sunscreen may not be necessary, but otherwise appropriate sun protective behaviours should be applied.
These guidelines may not guarantee sufficiency in everyone. For example, in Tasmania the prevalence of vitamin D deficiency (≤50 nmol/L) was still 43% among the subgroup of healthy adults who were most sun seeking (reported sun exposure of >4 hours a day during weekends and holidays).3 As a result, specific recommendations for Tasmania were developed (See factsheets at www.cancertas.org.au/healthy-living/sunsmart) particularly drawing attention to times of year where the UV index is moderate (late summer to early autumn and early to mid spring) because vigilance during this period can reduce the winter dip in vitamin D levels, which is of particular importance in Tasmania.
Modifications of advice for at risk groups
Vitamin D deficiency is common in the elderly. The prevalence of deficiency defined as serum 25(OH)D <50 nmol/L in a community dwelling population-based sample of people over the age of 50 years in Tasmania was 45%.36 Some elderly populations are at higher risk of vitamin D deficiency and potentially of poor bone health as a result of this deficiency. This includes elderly and/or disabled people in low and high level residential care;37 dark-skinned people,38 particularly migrants or if modest dress is worn;38 people with a disability or chronic disease, eg. multiple sclerosis;3 fair-skinned people and other people at risk of skin cancer who avoid sun exposure.39 For example, 86% of women and 68% of men in a sample of older people in residential aged care facilities in the northern Sydney area had a serum 25(OH)D level <28 nmol/L.40 Tailoring of sun exposure advice according to the specific requirements of these groups may be necessary. In particular, for dark-skinned individuals the duration of sun exposure to achieve adequate vitamin D levels is 3–6 times that described for fair-skinned people. In other high risk groups, eg. elderly in residential care,41 people with skin cancer or with conditions resulting in photosensitivity, increases in sun exposure may not be safe or feasible and supplements may be needed.
Who to test?
Population screening for vitamin D deficiency in older adults by measuring serum 25(OH)D is not recommended, but testing high risk groups is appropriate. This includes people with known osteopenia and primary or secondary osteoporosis and the at risk groups described. If access to testing is a substantial barrier to seeking treatment, it may be justified to treat adults who are at very high risk of vitamin D deficiency without testing to confirm deficiency. In this situation, it is suggested that the same dose used to treat moderate to severe deficiency is given.1
There is the potential for significant variability in test results, especially between laboratories, and at the analytically important range (<50 nmol/L) of the assay. Clinicians should be aware of the imprecision of current 25(OH)D testing and exercise caution when interpreting results in clinical practice.
Correcting vitamin D deficiency
Mild vitamin D deficiency may be corrected with increased sun exposure. However, where this is not possible or feasible, or in the case of moderate to severe deficiency, correction is best achieved by the use of vitamin D supplements. One suggested regimen is 3000–5000 IU vitamin D3 daily for 6–12 weeks, checking levels after 12 weeks. Most people will need ongoing treatment at a maintenance dose of 1000–2000 IU/day. If this does not correct deficiency, rule out underlying gastrointestinal disorders such as coeliac disease.
Higher intermittent doses of vitamin D have been used and will correct deficiency. This may be useful where adherence to treatment is a major problem. However, such preparations (containing 50 000 IU vitamin D3) are not routinely available in Australia and prescribing permission needs to be obtained from the Therapeutic Goods Administration (see www.tga.gov.au/hp/access-authorised- prescriber.htm#about) unless they are obtained from compounding chemists, although quality control is an issue in this context. Safety issues as described should also be considered before taking such an approach.
As low calcium intakes and associated hyperparathyroidism increases the degradation of vitamin D compounds, a daily intake of 1000–1300 mg calcium per day, preferably using calcium-rich foods should also be encouraged.
Conflict of interest: none declared.
References