Case study
Thea, aged 63 years, presents for review after a small inferior myocardial infarction (MI) 3 months previously. You note that her total cholesterol before starting a statin in hospital was high (7.1 mmol/L [normal range <5.5]) with an LDL of 5.5 mmol/L (normal range <3.4), an HDL of 0.9 mmol/L (normal range 1.0–2.2) and a triglyceride of 1.4 mmol/L (normal range <1.7). Two years previously her total cholesterol was marginally high at 5.6 mmol/L with an LDL of 4.0 mmol/L, an HDL of 1.0 mmol/L and triglyceride of 1.2 mmol/L. No specific treatment was instituted at the time apart from general lifestyle advice. Thea says she is feeling fine and has had no chest pain since the MI. However, she thinks the MI has 'slowed her down' a little. She has gained 'a lot of weight' over the past year or two (5 kg in the past year and 8 kg compared to 2 years ago). Her medications include aspirin 100 mg daily, carvedilol 6.25 mg twice per day, atorvastatin 20 mg daily, ramipril 10 mg daily, vitamin D 50 000 IU 3 monthly, ibuprofen 400 mg intermittently and a range of complementary medicines, vitamins and mineral supplements.
Thea's examination findings are:
- weight 91 kg, height 168 cm (body mass index [BMI] 32.2 kg/m2)
- blood pressure 148/95 mmHg
- pulse 52 bpm
- thyroid palpable.
You order a thyroid stimulating hormone (TSH), which is elevated at 82 mIU/L (normal range 0.3–3.0). You repeat this test with a serum free thyroxine (T4), which is reduced at 6.8 pmol/L (normal range 15–25) and a thyroid peroxidase (TPO) antibody level of 62 (<50 mIU/L). On repeat the TSH is 78 mIU/L.
You explain to Thea that she has hypothyroidism, most likely caused by autoimmune chronic lymphocytic thyroiditis. She volunteers that her mother had an underactive thyroid and one of her maternal aunts had an overactive thyroid in her youth.
Question 1
What dose of thyroxine would you use?
Question 2
When will you recheck her thyroid function and what tests will you order for monitoring?
Question 3
Are any of Thea's usual medications likely to be affected by her thyroxine replacement?
Case study continued
Thea starts and gradually increases thyroid replacement therapy and 6 weeks after maintaining a thyroxine dose of 150 μg/day her thyroid function tests (TFTs) show:
- T4 23 pmol/L (normal range 15–25)
- TSH 2.4 mIU/L (normal range 0.3–3.0).
Question 4
When would you recheck Thea's thyroid replacement therapy?
Case study continued
You recheck Thea's TFTs 6 months later and find that they are stable. Six months after that (1 year after adequate replacement was started) her TFTs show:
- T4 16 pmol/L (normal range 15–25)
- TSH 12 mIU/L (normal range 0.3–3.0).
Question 5
What might have happened since her last test?
Case study continued
Thea went to a thyroid support group meeting where several people explained that they were 'resistant' to the effects of T4 and needed T3 to get the beneficial effects of replacement therapy. She asks if she should try T3 to see if she feels better on it.
Question 6
How will you address her concerns?
Answer 1
The final replacement dose is usually 1.6 µg/kg/ day or between 50 and 200 µg/day. However, it is important to start low and increase slowly, particularly in Thea's case due to her age and comorbidity (ischaemic heart disease). Thea could start with a dose of 25 µg/day (half of a 50 µg tablet), increasing by 25 µg/day increments at 2 weekly intervals until the full replacement dose is reached, as long as there are no signs of adverse cardiovascular effects. As her weight is 91 kg, her final dose would be expected to be around 150 µg/ day. This may change after checking her thyroid function on treatment.
Answer 2
When thinking about monitoring thyroid therapy it is important to consider the function of the hypothalamic pituitary system as well as the thyroid. In primary hypothyroidism, the hypothalamic/pituitary system also becomes functionally 'hypothyroid' and takes time to recover and respond to the rising levels of thyroxine. Thea's hypothyroidism has probably been present for 1–2 years and she is now profoundly hypothyroid with a very high TSH. However, gradual replacement as described above should allow plenty of time for her system to recover. The half-life of T4 is 1 week. Generally, it is thought that six half-lives of the medication are required before levels reach a steady state. Thea's thyroid function should be checked 6 weeks after starting the full replacement dose.
In primary hypothyroidism, TSH levels are the most useful test for monitoring. Free T4 is usually ordered as well, however, TSH is thought to be the most accurate measure. After commencement of treatment the TSH should gradually decrease into, but not below, the reference range. Free T3 is not useful in diagnosis or in monitoring replacement and is not usually provided by the laboratory. But it is useful to note that with therapeutic thyroxine therapy, free T3 levels tend to be slightly lower and free T4 levels slightly higher than normal. This is because the thyroid normally produces all the circulating T4 and 25% of the circulating T3, with most of the T3 produced in the tissues by conversion of T4 to T3. In therapeutic thyroid replacement, only T4 is given and then subsequently converted to T3 in the tissues resulting in lower levels of serum T3. With adequate replacement the TSH will vary around the middle of the normal range, the free T4 will be high normal or high and the free T3 will be low normal or low (Figure 1).1,2
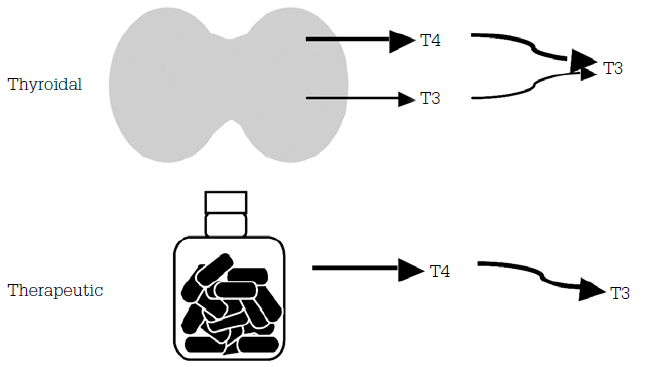
Figure 1. Metabolism of T3 and thyroidal and therapeutic T41,2
Answer 3
Many medicines can affect thyroxine levels (see the article 'Hypothyroidism' by So et al in this issue of AFP).3 In addition, the clearance of some drugs can be increased by the metabolic effects of thyroxine. In Thea's case, her beta-blocker (carvedilol) levels could be affected. While she was hypothyroid, levels of these medications would have been higher than predicted and these higher levels may explain part of her bradycardia (heart rate 52 bpm) despite only a low dose of carvedilol. Of course, this is also a symptom of hypothyroidism. As her hypothyroidism is treated, medication levels will decrease and her cardiologist might like to review her and consider increasing the dose of her betablocker.
As a general rule, medication levels increase/ decrease with hypo-/hyper-thyroidism and doses should be increased/decreased accordingly. One important exception to this rule is warfarin. This is because the effects of thyroxine on the production and clearance of clotting factors outweigh any effects on warfarin metabolism. With hypothyroidism, production is decreased but clearance is very much more decreased and warfarin dose needs to be increased. In hyperthyroidism, the opposite occurs with clotting factor production increasing but clearance is very much more increased and warfarin dose needs to be decreased. Anticoagulation can change dramatically with the introduction of thyroid therapy and INR levels need to be closely monitored.4
Answer 4
Once the patient is on full thyroxine replacement and TFTs are stable, an annual check is all that is required unless there is a dose adjustment or under/over replacement is clinically suspected. If dose adjustment occurs a repeat test is not usually needed until 3 months later.
In some patients, functioning thyroid tissue remains and is still secreting some thyroid hormone, but this slowly decreases as ongoing thyroid destruction occurs from the autoimmune thyroid disease. In these cases, thyroxine replacement may need to be increased as the disease progresses. But these patients tend to present with milder initial hyperthyroidism (eg. TSH 15 mIU/L). As Thea had severe initial hypothyroidism, she may well remain adequately replaced indefinitely on her final dose of T4 (150 µg/day).
Answer 5
Possible explanations include poor compliance, malabsorption due to a new gastrointestinal disease, starting a medication known to reduce thyroxine absorption or to increase clearance (see the article by So et al in this issue of AFP)3 or problems with thyroxine storage. As Thea has autoimmune thyroid disease, she is at an increased risk of having coeliac disease as part of an 'autoimmune cluster',5 so these conditions are important to consider. Also, she may be taking a supplement containing calcium or iron. If so, she should either cease taking it or separate the thyroxine and metal therapy by several hours (eg. taking one in the morning and one in the evening). Thyroxine is affected by heat and humidity and storage problems were common when tablets were dispensed in bottles. Thyroxine tablets are now dispensed in blister packs with instructions to keep the strip in use out of the refrigerator and the rest of the strips in the refrigerator.
Answer 6
The issue of T4 versus T3 has been debated in many professional forums and the consensus seems to be that objectively, T4 therapy is better than T3 therapy.6 T3 therapy has significant disadvantages because of its short half-life (1 day compared to 1 week for T4) and daily T3 use is associated with peaks and troughs rather than the flatter profile of T4 therapy. Prescribing T3 in several divided doses through the day does flatten the profile but this is inconvenient and may not be adhered to. Research has shown that no benefit of combined levothyroxine and triiodothyronine (T3) therapy.6
Conflict of interest: none declared.