Causes of thyrotoxicosis
Table 1 outlines the various causes of thyrotoxicosis. The most common cause is Graves disease followed by toxic multinodular goitre, the latter increasing in prevalence with age and iodine deficiency.3,4 Other important causes include toxic adenoma and thyroiditis. Exposure to excessive amounts of iodine (eg. iodinated computed tomography [CT] contrast media, amiodarone) in the presence of underlying thyroid disease, especially multinodular goitre, can cause iodine induced thyrotoxicosis. Thyroiditis is a condition that may be suitable for management in the general practice setting. Patients should be monitored for the hypothyroid phase, which may occur with this condition. Referral to an endocrinologist is recommended for the management of thyrotoxicosis if thyroiditis is unlikely or has been excluded.
Table 1. Overview of causes of thyrotoxicosis3,4,6,28,29,30
| Aetiology | Pathogenesis | Clinical presentation and course of disease | Radionuclide thyroid scan | Laboratory results | Treatment |
|---|
| Common |
|---|
| Graves disease |
TSH receptor Ab increases thyroid hormone production and causes thyroid hyperplasia |
Female:male ratio 5–10:1
Peak onset 40–60 years
Diffuse, usually symmetrical goitre
Graves ophthalmopathy
Associated with other autoimmune diseases |
Normal or elevated diffuse uptake pattern* |
TSH receptor Ab positive
TPO Ab often positive |
Antithyroid drugs first line for first time presentations. RAI and less commonly thyroidectomy are alternatives. Patient factors and preference guide therapy |
| Toxic multinodular goitre and Toxic adenoma |
Nodule autonomy |
Female > male
Onset usually:
- 50+ years (TMNG)
- 30–50 years (TA)
Nodular goitre often present for years (TMNG)
Slowly growing solitary thyroid nodule, usually >3 cm (TA) |
Normal or elevated multifocal (TMNG) or focal (TA) uptake with suppression of surrounding thyroid uptake* |
TPO Ab low titre or absent |
Remission is rare without definitive treatment. RAI is first line. Surgery for compressive symptoms, large goitre, coexisting thyroid cancer or hyperparathyroidism. Occasionally long term low dose carbimazole is required |
| Painless, postpartum thyroiditis |
Autoimmune: destruction of thyroid follicles with release of stored thyroid hormone |
Typically 1–6 months after delivery
Diffuse, small goitre
Thyrotoxicosis for 1–2 months often followed by hypothyroidism for 4–6 months; hypothyroidism may be permanent (20%)
Common in women with type 1 diabetes |
Near absent uptake |
TPO Ab high titre in most Normal ESR |
Beta-blocker for symptoms Thyroxine if the hypothyroid phase is prolonged, symptomatic, if breastfeeding or attempting further pregnancies |
| Exogenous thyroid hormone |
Excess ingestion of thyroid hormone Iatrogenic, intentional, or factitious |
Usually no goitre |
Near absent uptake |
TPO Ab low titre or absent
Low Tg levels |
Cease/reduce thyroid hormone as appropriate |
| Less common |
|---|
| Painless sporadic thyroiditis |
Autoimmune: destruction of thyroid follicles with release of stored thyroid hormone |
Female:male ratio 2:1
Sporadic, cases peak at 30–40 years of age
Diffuse, small goitre
Thyrotoxicosis for 1–2 months often followed by hypothyroidism for 4–6 months; hypothyroidism may be permanent (20%) |
Near absent uptake |
TPO Ab high titre in most Normal ESR |
Beta-blocker for symptoms Thyroxine if the hypothyroid phase is prolonged or symptomatic |
| Painful subacute thyroiditis |
Possibly caused by a viral infection. Destruction of thyroid follicles with release of stored thyroid hormone |
Female:male ratio 5:1
Peak onset 20–60 years of age
Often follows an upper respiratory tract infection
Tender goitre
Thyrotoxicosis for 1–2 months often followed by hypothyroidism for 4–6 months; hypothyroidism may be permanent (5%) |
Near absent uptake |
TPO Ab low titre or absent
ESR almost always >50 mm/hr
Normal or increased WBC |
Beta-blocker for symptoms
Nonsteroidal anti-inflammatory drugs for thyroid pain
Glucocorticoids may be required for more severe pain
Thyroxine if the hypothyroid phase is prolonged or symptomatic |
| Amiodarone induced thyroiditis |
Type 1 – excess iodine
Type 2 – destructive thyroiditis |
Type 1 – underlying thyroid disease present.
More common in iodine deficient areas, diffuse or nodular goitre
Type 2 – no underlying thyroid disease, normal gland or small goitre
Can present up to a year after ceasing amiodarone |
Usually low uptake and not discriminatory
Uptake occasionally seen in type 1 thyroiditis |
TSH receptor Ab may be present in type 1 thyroiditis if there is underlying Graves disease |
Type 1 – antithyroid drugs
Type 2 – corticosteroids
Thyroidectomy may be required
Can be difficult to distinguish between type 1 and 2 thyroiditis |
| ESR = erythrocyte sedimentation rate; RAI = radioactive iodine therapy; TA = toxic adenoma; TMNG = toxic multinodular goitre; TPO Ab = thyroid peroxidase antibody; Tg = thyroglobulin; TSH receptor Ab = thyroid stimulating hormone-receptor antibody; WBC = white blood count. * Uptake may be low in iodine induced thyrotoxicosis |
Clinical features
The most frequent symptoms of thyrotoxicosis are nervousness, heat intolerance, palpitations, fatigue and weight loss (note: weight gain occurs in 10% of people).3 Common examination findings include agitation, sinus tachycardia, fine tremor and hyper-reflexia.3 There is some correlation between the clinical severity and the degree of thyroid hormone excess, but this varies substantially between individuals.4 Elderly patients often present with nonspecific symptoms. However, of the elderly patients with hyperthyroidism, up to 20% will have atrial fibrillation.5
Graves disease
Graves disease is an autoimmune disorder characterised by the presence of thyroid stimulating hormone (TSH) receptor antibodies. It can occur at any age, but has a peak onset between 40 and 60 years.6 Women are 5–10 times more likely to be affected than men.6 It clusters in families and genetic associations have been found, but no single gene is known to be necessary or sufficient to cause Graves disease.6,7 Smoking, psychological stress and the postpartum period are associated with the development of Graves disease.6,7 Other autoimmune diseases, such as coeliac disease, occur more frequently in patients with Graves disease and this risk persists after treatment.8
Patients with Graves disease have thyrotoxicosis associated with a diffuse goitre. Clinical features that distinguish Graves disease from other causes of thyrotoxicosis include the presence of Graves ophthalmopathy (thyroid eye disease) and the presence of uncommon manifestations of Graves disease such as thyroid dermopathy (pretibial myxoedema, 1–2%) and thyroid acropachy (digital clubbing, <1%).3 Clinical features of Graves ophthalmopathy occur in about 50% of patients with Graves disease and a further 20% have evidence of ophthalmopathy on imaging.9 Eyelid lag or retraction and periorbital oedema are the most frequent signs and proptosis is common.9
Evaluation of thyrotoxicosis
Thyroid function tests
Serum TSH is an exquisitely sensitive indicator of thyroid status in patients with an intact hypothalamic pituitary axis and should be used as the initial screening test for thyrotoxicosis.4 A low TSH should prompt testing of free thyroid hormone concentrations (Figure 1). When the TSH is normal, it is rare that a patient is thyrotoxic.4
In overt thyrotoxicosis, TSH is suppressed (<0.01 mIU/L) and free thyroxine (T4) and free triiodothyronine (T3) are increased. Triiodothyronine (T3) thyrotoxicosis describes TSH suppression with elevated free T3 but normal free T4. Subclinical hyperthyroidism is defined by a low or suppressed TSH in the presence of normal free thyroid hormone concentrations (both free T3 and free T4). 'Subclinical' is a somewhat misleading term as typical clinical features of thyrotoxicosis may occur in subclinical hyperthyroidism.4
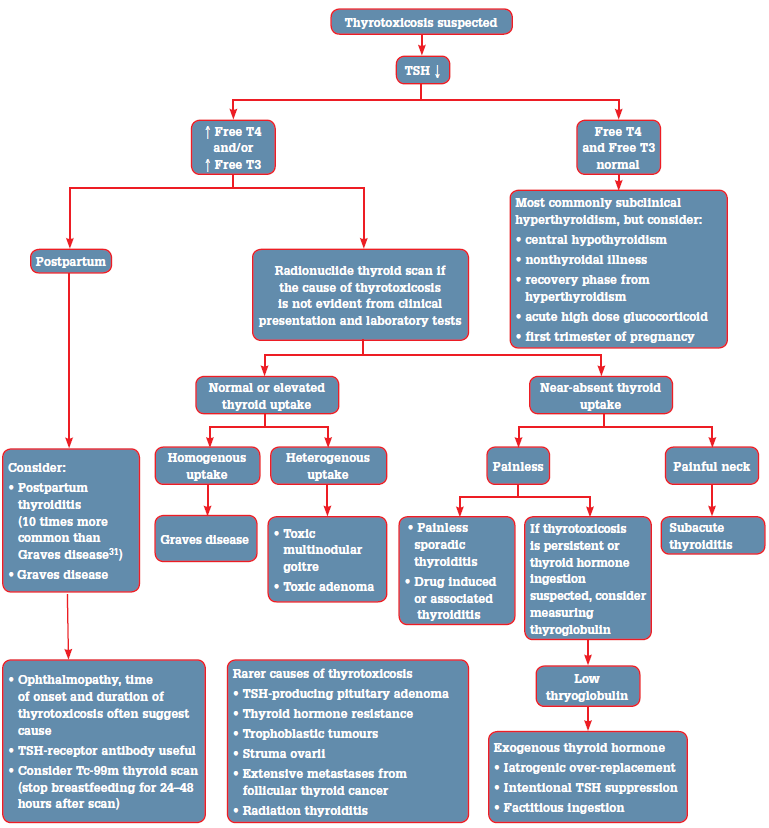
Figure 1. Evaluation of suspected thyrotoxicosis
Thyroid autoantibodies
Measurement of TSH receptor antibodies is useful to establish the diagnosis of Graves disease, especially when a radionuclide thyroid scan is not able to be performed (eg. in pregnancy, lactation), when the presentation is atypical (eg. euthyroid Graves ophthalmopathy), or in amiodarone induced thyrotoxicosis.10 Thyroid stimulating hormone receptor antibodies may either have a stimulating effect (stimulating antibody) or an inhibitory effect (blocking antibody) on the TSH receptor. Most assays can't distinguish between stimulating and blocking antibodies, but the functional status of the patient is known from thyroid function tests. Up to 10% of patients with Graves disease have undetectable TSH receptor antibodies, probably due to inadequate sensitivity of the assay (Table 2).10
Thyroid peroxidase and thyroglobulin autoantibodies are less specific, but may be useful in the setting of thyroiditis.
Table 2. Prevalence of antithyroid antibodies1,10,32
| | Thyroperoxidase autoantibodies | Thyroglobulin autoantibodies | TSH receptor antibody |
|---|
| General population |
8–27%
(11% without history of thyroid disease in an Australian cohort*) |
5–20%
(5% without history of thyroid disease in an Australian cohort*) |
1–2%
(significance of these positive values remains to be determined) |
| Graves disease |
50–80% |
50–70% |
90–99%† |
| Chronic autoimmune thyroiditis |
90–100% |
80–90% |
10–20% |
* The Busselton Thyroid Study1
† Second generation TSH receptor antibody assays using human TSH receptor coated tubes have a sensitivity of 90–99% and specificity of 95–100% for Graves disease10 |
Imaging
If the aetiology of the thyrotoxicosis is not evident from the clinical presentation and laboratory tests, a radionuclide thyroid scan should be performed. When the presentation is sufficient to diagnose Graves disease – symmetrically enlarged goitre, recent onset ophthalmopathy and moderate to severe thyrotoxicosis – a clinical diagnosis can be made without further investigation.4 Technetium (Tc-99m) pertechnetate is the main diagnostic radionuclide used for thyroid scans in Australia and has an effective dose of 2.4 millisieverts (mSv), comparable to the annual dose of natural background radiation and similar to CT imaging (eg. head 2 mSv, chest 7 mSv).11 It is contraindicated in pregnancy, and breastfeeding women should discontinue breastfeeding for 24–48 hours following a scan.
Ultrasound does not usually aid in distinguishing the cause of thyrotoxicosis.
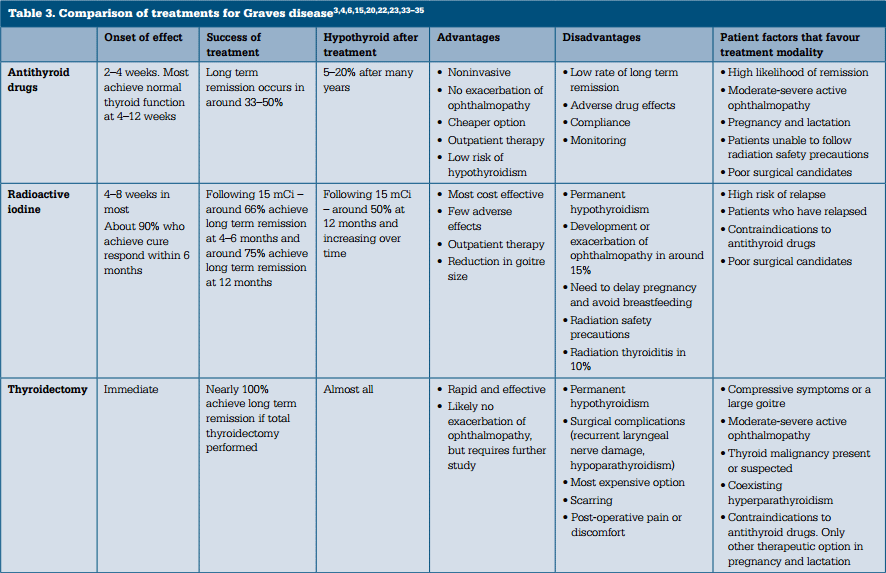
Management of Graves disease
There are three treatment options for Graves disease (Table 3):
- antithyroid drugs
- radioactive iodine (I131)
- thyroidectomy.
The characteristics of an individual patient influence the relative potential benefits and harms of these treatments. Antithyroid drugs are the initial therapy of choice for the majority of patients with Graves disease treated in Australia. Definitive therapy with radioactive iodine is indicated in patients who relapse following a course of antithyroid drug therapy.
Symptomatic treatment
Beta-blockers may be used for symptom control before the onset of antithyroid drug effect. The various beta-blockers are similarly effective in improving the adrenergic symptoms of thyrotoxicosis (eg. palpitations, tachycardia, tremor, anxiety and heat intolerance).3 A nondihydropyridine calcium channel blocker can be used to control heart rate when beta-blockers are not tolerated or contraindicated (eg. asthma).4
Antithyroid drugs
The thionamide antithyroid drugs carbimazole and propylthiouracil decrease thyroid hormone synthesis by inhibiting thyroid peroxidase.12 Carbimazole is the first line thionamide in almost all patients as it results in a more rapid improvement in thyroid hormone levels, has less hepatotoxicity, and can be given once daily due to its longer half life.4,12 Propylthiouracil is the preferred antithyroid drug in the first trimester of pregnancy, in the treatment of thyroid storm (also inhibits the conversion of T4 to T3), and in patients with minor reactions to carbimazole where radioactive iodine or surgery is not appropriate.4
Agranulocytosis (neutrophil count <0.5x109/L) is a rare but lifethreatening complication of both antithyroid drugs with an incidence of 0.2–0.5% (Table 4).4,12 Although it most often occurs during the first 3 months of treatment, it may occur at any time.12,13 Patients should be educated to suspend antithyroid therapy and obtain a neutrophil count if they develop mouth ulcers, fever, sore throat or other symptoms suggestive of infection. Routine blood counts are of limited clinical utility and are not cost effective.4 Due to cross reactivity between the two antithyroid medications, agranulocytosis with one drug is an absolute contraindication to trialling the other.4,12
Severe hepatocellular injury occurs with propylthiouracil in 0.1% of patients treated with the drug, and approximately 10% of these patients develop liver failure resulting in either a liver transplant or death.14
The recommended starting dose of carbimazole is 10–30 mg/day in 2–3 divided doses depending on severity of thyrotoxicosis, although larger doses may be used in severe disease.15 Four weeks following initiation of therapy, clinical review with repeat thyroid function tests should be undertaken to avoid hypothyroidism. Antithyroid drug therapy is tapered to a maintenance dose (usually carbimazole 2.5–10 mg) and ceased after 12–18 months of therapy.15,16
The rate of long term remission with antithyroid medications in Australia is less than 50%.15,17 Male gender, age <40 years, a large goitre, marked elevation of T4 and T3 and possibly a high titre of TSH receptor antibody, are factors associated with a lower rate of remission.18,19 Approximately 5–20% of patients in remission eventually develop hypothyroidism due to autoimmune thyroiditis or the presence of TSH receptor blocking antibodies.20
Occasionally, free T3 concentrations remain increased despite normal or even low free T4 concentrations (T3 predominant Graves disease), and in this situation free T3 is a useful guide to dosing.12 Serum TSH concentrations may remain suppressed for several months after normal free thyroid hormone levels are established, so TSH is not a good biomarker to guide drug therapy in the early stages.12
Table 4. Adverse effects of antithyroid medication3,4,12
| Common (1–10%) | Practice points |
|---|
- Gastrointestinal effects (nausea, vomiting, gastric discomfort) (CBZ, PTU)*
|
- Dose dependent, use divided doses of CBZ initially
|
- Rash (urticarial or macular) (CBZ, PTU)
|
- Exclude vasculitis
- Minor reactions may resolve with antihistamine while antithyroid drug therapy is continued
|
- Arthralgia or fever (CBZ, PTU)
|
- Discontinue drug as this may be indicative of more severe immunological side effects
- If fever, exclude agranulocytosis
|
- Transient mild neutropaenia
|
- Monitor to ensure agranulocytosis does not develop
|
| Uncommon/rare but severe | Patient information |
|---|
- Agranulocytosis (0.2–0.5%) (CBZ, PTU)
- Hepatocellular liver injury (PTU)
- Cholestatic hepatitis (CBZ)
- Aplasia cutis and choanal or oesophageal atresia (CBZ)
- Polyarthritis (CBZ, PTU)
- ANCA-positive vasculitis (PTU>CBZ)
|
- Patients should be informed to report to their doctor if they develop:
- fever, mouth ulcers, sore throat or other symptoms suggestive of infection (suspend drug and urgently report to obtain neutrophil count)
- severe fatigue, nausea, abdominal pain, jaundice, dark urine or pale stools (suspend drug and urgently report for investigation)
- rash
- arthralgia
|
| Baseline blood tests |
- Full blood count
- Liver function tests
|
| * CBZ = carbimazole; PTU = propylthiouracil |
Radioactive iodine
Following oral administration, radioactive iodine is transported into thyroid follicular cells resulting in cell necrosis over weeks to months.21 The cure rate (euthyroid or hypothyroid) following the administration of a 15 mCi dose of radioactive iodine for Graves disease is 67–81% at 12 months, with hypothyroidism occurring in most and increasing with time.22,23 Patients with a large goitre and more severe thyrotoxicosis are more likely to require a second dose of radioactive iodine, usually given 6–12 months after the initial treatment.3,24
Radioactive iodine therapy is generally well tolerated. However, radiation thyroiditis occurs in approximately 10% of patients with transient worsening of thyrotoxicosis and painful thyroid inflammation in some.21,25 Antithyroid drugs are generally used before the administration of radioactive iodine to promptly achieve euthyroidism and to attenuate exacerbation of thyrotoxicosis should thyroiditis occur.12 Following radioactive iodine therapy, women should avoid pregnancy for 6 months to ensure stable euthyroidism; men should allow 4 months for turnover of sperm production.4 For several days following radioactive iodine treatment, patients are advised to adhere to radiation safety precautions to prevent unnecessary radiation exposure to others. This includes avoiding close contact with children. Radioactive iodine therapy is not recommended in the presence of moderate to severe active Graves ophthalmopathy as it can exacerbate the eye disease.4
Surgery
Thyroidectomy results in rapid control of thyrotoxicosis and has minimal risk of recurrence when a total thyroidectomy is performed.26 Antithyroid drugs should be initiated before surgery to reduce the risk of thyroid storm.4 With experienced surgeons, the risk of permanent hypoparathyroidism is <2% and permanent recurrent laryngeal nerve injury is <1%.4
Graves ophthalmopathy
Treatment for Graves ophthalmopathy includes local measures, corticosteroids, orbital radiation and surgery.4 Smoking and radioactive iodine are risk factors for the development or progression of Graves ophthalmopathy.9,27 Prednisolone prophylaxis is effective in patients with mild active ophthalmopathy receiving radioactive iodine.27
Pregnancy
Carbimazole during pregnancy has been associated with birth defects, including aplasia cutis and 'carbimazole embryopathy', characterised by choanal atresia or oesophageal atresia.14 Therefore, during pregnancy it is recommended that propylthiouracil be used in the first trimester and then changed to carbimazole in the second trimester.4 Antithyroid drugs can be stopped in about 30% of women by the third trimester.3 Thyroid stimulating hormone receptor antibodies are measured during pregnancy as this can predict the risk of neonatal Graves disease.4 Women with a history of Graves disease are at an increased risk of relapse or thyroiditis in the postpartum period.12
Key points
- TSH concentration should be used to screen for thyrotoxicosis.
- It is important to determine the underlying cause of thyrotoxicosis in order to guide management:
- a radionuclide thyroid scan has the highest diagnostic yield
- TSH receptor antibodies are useful, especially in certain clinical scenarios
- other thyroid autoantibodies are less helpful, except when thyroiditis is present
- a thyroid ultrasound is seldom useful in this context.
- Antithyroid drugs, radioactive iodine and surgery are the therapies available for the management of Graves disease. The choice of therapy should be tailored to the characteristics of the individual patient.
Case study
Linda, aged 32 years, presented with 2 months of palpitations, tremor, heat intolerance, loose bowel motions and insomnia. She had lost 20 kg, but attributed this to diet and attendance at 'boot camp'. On examination, her pulse was 120/min and regular and her blood pressure was 130/80 mmHg. She was agitated, had a fine tremor, warm moist palms, and was hyperreflexic. There were no signs of ophthalmopathy. She had a small to moderate sized diffuse goitre (Figure 2) and a bruit was present. The remainder of the examination was normal.
Linda's thyroid function test results showed:
TSH: <0.01 mIU/L (normal range 0.5–4.0 mIU/L)
T4: 58 pmol/L (normal range 10–25 pmol/L)
T3: 23 pmol/L (normal range 3.1–5.4 pmol/L).
Other blood tests showed alkaline phosphatase 135 U/L (30–120 U/L) and a normal full blood count (FBC). A thyroid scan demonstrated diffuse increased uptake of 6.1% (Figure 3). Linda was diagnosed with Graves disease and commenced carbimazole 10 mg twice per day and propranolol 20 mg three times daily.
At 4 week review her thyroid function tests demonstrated:
TSH: <0.01 mIU/L
T4: 28 pmol/L
T3: 11 pmol/L.
Her carbimazole dose was reduced to 10 mg in the morning.
During Linda's seventh week of treatment she developed a fever, mouth ulcers and symptoms of gastroenteritis. A FBC revealed agranulocytosis (neutrophils 0.21 x 109/L). She was admitted to hospital and received broad spectrum intravenous antibiotics and granulocyte colony stimulating factors (G-CSF). Following recovery of her neutrophil count she underwent thyroidectomy. Her surgery was uncomplicated and she was discharged on thyroxine.
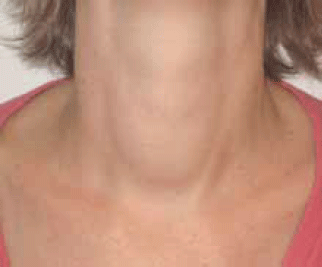
Figure 2.
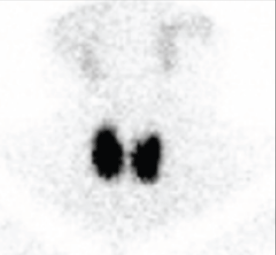
Figure 3.
Conflict of interest: none declared.