Lower urinary tract dysfunction, such as benign prostatic hyperplasia (BPH), overactive bladder (OAB) and urinary incontinence, are common conditions that adversely affect health-related quality of life and increase the risk of institutionalisation.1 A Deloitte Access Economics report commissioned by the Continence Foundation of Australia found that more than 4.2 million Australians aged 15 years and older had urinary incontinence in 2010.2 The prevalence of urinary incontinence increases with age and it is estimated that more than one in four men aged over 70 years have urinary incontinence.1 The presence of poor general health, medical comorbidities, severe physical limitations, cognitive impairment, neurological conditions, recurrent urinary tract infection (UTI) and prostatic diseases have been associated with urinary incontinence.1
Persistent, non-neurogenic urinary incontinence in adult males can be classified as stress urinary incontinence (SUI), OAB with urge urinary incontinence (UUI), mixed incontinence, overflow (paradoxical) incontinence, continuous urinary incontinence (eg fistula) and functional incontinence.1,3 This article briefly reviews the current understandings of the pathophysiological mechanisms in SUI and OAB/UUI, and offers a set of practical, action-based recommendations and treatment strategies. All treatment options should be discussed to facilitate informed decision-making, and these options can then be individualised on the basis of patient preference, current comorbidities and specific circumstances.
Methods and evidence synthesis
A literature search was performed on the PubMed database for English-language, original and review articles published up to December 2016. Keywords included ‘male stress urinary incontinence’, ‘overactive bladder’ and ‘urge incontinence’. This article was formulated from a clinical review of contemporary literature; a detailed analysis of all relevant studies is not the goal of this article.
Pathophysiological mechanisms
Male stress urinary incontinence
The main pathophysiology behind SUI in men relates to underlying dysfunction of the urethral sphincter complex and/or change in urethral axis. This is often a complication following prostate surgery, such as radical prostatectomy or transurethral resection of prostate (TURP). Other causes of male SUI are iatrogenic sphincter injury (eg sphincterotomy in spinal patients), neurological conditions or trauma to the pelvic floor (eg pelvic trauma in motor vehicle accident). The exact incidence of SUI may vary depending on the underlying pathology, definition of SUI and source of data (eg physician versus patient report).4 The expected benefits of robotic prostate surgery in reducing the urinary and sexual side effects following prostatectomy have not yet been demonstrated conclusively in recent literature.5 Reported risk factors for post-prostatectomy SUI include the patient’s:5
- age
- body mass index
- pre-operative bladder function and urinary continence status
- prior radiation therapy
- pre-operative length of membranous urethra
- prior TURP
- vascular comorbidities
- stage of disease
- surgical technique employed, including nerve sparing
- surgeon’s level of experience.
While sphincter deficiency is often the main causative factor,4 other bladder conditions, such as detrusor overactivity, poor bladder compliance and detrusor underactivity, can often co-exist and contribute to the pathophysiology of SUI. Anastomotic stricture and scarring of the urethral tissue due to surgery and/or radiation should be considered in a patient who complains of urinary incontinence and decreased urine flow.
Male overactive bladder and urge urinary incontinence
OAB is a clinical syndrome characterised by urinary urgency, with or without urge incontinence, usually accompanied by frequency and nocturia.6 Proposed pathophysiological mechanisms include age-related changes in smooth muscle, leading to:
- hyper-excitability of muscarinic receptors in the detrusor smooth muscle, urothelium and neurovascular structures, and atropine resistance
- increased afferent (sensory group C fibres) nerve activity and hypersensitivity of other ion channels7
- denervation at the spinal and cortical levels, resulting in hyperactive voiding that is secondary to spinal micturition reflexes.
Other neurological conditions, such as Parkinson’s disease, multiple sclerosis or stroke, may cause loss of inhibitory neurons, resulting in neurogenic voiding dysfunction.7
The risk factors for UUI include neurological conditions, various inflammatory processes of the bladder, bladder outlet dysfunction, physiological ageing and psychosocial stressors, or the condition may be idiopathic in nature.8 Although it is accepted that OAB occurs more commonly in women, the true prevalence of OAB in men remains largely unknown. This is because most storage symptoms are frequently attributed to an enlarged prostate. The most common finding in patients with UUI is detrusor overactivity, which is a urodynamic observation of involuntary bladder contractions that are commonly associated with a corresponding sensation of urgency during bladder filling. Enlarged prostate and ensuing bladder outlet obstruction can result in bladder adaptations and abnormal bladder contraction (ie detrusor overactivity). It is also important to exclude other conditions that can simulate OAB-like symptoms, such as UTI, bladder stones and carcinoma in situ.7–10 Ageing increases the prevalence of UUI and SUI, and the two can often co-exist, leading to mixed incontinence.
Practical approach to diagnosis
Basic clinical evaluation should include comprehensive history-taking, focused physical examination, urinalysis and post-void residual measurement (Table 1).1,11–13 Evaluation of male urinary incontinence should identify the types of incontinence (eg stress, urge, mixed), with an emphasis on the timing and severity of the incontinence, and overall impact on quality of life. The presence of other urinary symptoms and past urological conditions or surgery provide useful information during the clinical assessment of the patient. Other relevant medical conditions, such as any neurological conditions, diabetes, previous pelvic injury and cognitive impairment, should also be assessed. Identifying the most bothersome symptom will often help direct management. The use of validated patient questionnaires, such as the International Consultation on Incontinence Questionnaire (ICI-Q), can often provide symptom clarification and serve as a marker for improvement. A three-day frequency–volume chart or bladder diary (eg indicating daytime and night-time frequency of micturition, episodes of incontinence, voided volumes, 24-hour urine output), is often very useful in men who report mixed incontinence.
Physical examination should include an abdominal examination to detect any abdominal or pelvic mass (eg palpable bladder), perineal examination for sensory loss, digital rectal examination for prostate size and nodules, and pelvic floor tone. A focused neurological examination is useful in screening for upper (eg multiple sclerosis, Parkinson’s disease) or lower (eg sacral nerve root lesion) motor neuron diseases. Urinalysis and microscopy are essential to exclude a UTI, while measurement of post-void residual urine offers a good estimate of voiding efficiency. A pad test (ie weighing the pad to measure the volume of urinary incontinence) can diagnose the severity of urinary incontinence and may be used to indicate treatment outcome. Blood tests for renal function are recommended if compromised renal function is suspected, and in cases of polyuria (in the absence of diuretics use), as documented by the frequency–volume chart, glycaemic index should be assessed.
Table 1. Assessment of urinary incontinence
|
Stages of assessment
|
Summary of key points
|
|---|
|
History
|
- Genitourinary system
- Sexual function
- Other relevant medical history
- Medication history
- Functional status and impact of incontinence on quality of life
|
|
Physical examination
|
- General status
- Abdominal, pelvic and rectal examination
- Relevant neurological examination
|
|
Initial tests
|
- Urinalysis
- Bladder diary
- Bladder scan for postvoid residual
- Renal function, fasting glucose level and prostate specific antigen test
- Imaging of renal tract
|
|
Specialised tests
|
- Cystoscopy
- Urodynamic study
|
|
Imaging studies, such as renal tract ultrasound, provide useful information in excluding the presence of upper urinary tract dilatation and co-existing bladder pathology (eg stones, tumours). Contrast studies, such as cysto-urethrography or computed tomography (CT), could assist in the identification of fistulas, strictures, bladder diverticulae or tumours. Specialised tests should be individualised with the use of cystoscopy to evaluate the presence of urethral strictures, an obstructive prostate, bladder stones or tumours.
A pressure–flow study provides valuable information on detrusor function. Urodynamic studies have a role in patients with suspected voiding difficulties or neuropathy, failed treatment, or those considering surgical treatment. Urodynamic studies provide a physiological assessment of bladder and outlet function, and demonstrate dyssynergia of bladder contraction and outlet opening, such as seen in bladder denervation.13 Referral to a urologist is recommended for men with a provisional diagnosis other than BPH or OAB and those with a known history of haematuria, neurological or prior genitourinary surgery, radiation or trauma (Box 1).
Box 1. Red flags for referral to urologist
- Uncertain diagnosis and inability to develop a reasonable management plan
- Lack of response to an adequate trial of conservative therapies (eg bladder training, pelvic floor muscle therapy, drug therapy)
- Haematuria without infection and/or abnormal urine cytology
- Complex medical history, including the presence of neurological condition (eg multiple sclerosis, spinal cord lesions, cerebrovascular disease)
- Abnormally high postvoid residual urine volume
- Prostate nodule or family history of prostate cancer
- History of pelvic or prostate surgery and/or radiation therapy
|
Role of the general practitioner
Continence assessment includes identifying the type of incontinence (ie urgency, stress-related, mixed), the severity (number and size of pads used, preferably pad weights) and the impact on activity or quality of life. In patients who have mixed incontinence, such as urgency and stress incontinence, it is important to determine which is more bothersome. In the presence of complicated lower urinary tract dysfunction, symptoms such as haematuria, recurrent UTIs, dysuria and pain will require further investigation or specialist referral to exclude malignant or infectious pathology. Medical comorbidities, especially conditions such as diabetes, ischaemic heart disease or congestive cardiac failure, neurological conditions, chronic pulmonary disease and obesity, can exacerbate OAB and SUI symptoms. Treating these conditions may not eliminate incontinence, but it may lessen the severity.
Initially the general practitioners (GPs) should order urine microscopy and culture to exclude infection, haematuria and pyuria. The patient should be advised to keep a bladder diary to record the number and time of voids in a 24-hour period, volumes voided, incontinence episodes, fluid intake, degree of urgency and incontinence over a three-day period. The bladder diary allows documentation of functional bladder capacity, and checks for nocturnal polyuria (where nocturnal voided volume is >33% of the 24-hour volume) and incontinence. Post-void residual urine – often detected by bladder ultrasonography – is useful to check for incomplete emptying, which may suggest the presence of outlet obstruction or an underactive bladder (urinary retention). Simple blood tests to evaluate renal function, blood glucose level and prostate-specific antigen (PSA) may be indicated in symptomatic men.
Treatment strategies
Initial management of male urinary incontinence usually consists of basic diagnostic investigations to exclude any reversible conditions, such as a UTI (Figure 1). Conservative, non-invasive treatment options include lifestyle interventions, pelvic floor muscle training (PFMT) with or without biofeedback, and bladder retraining.9 Lifestyle interventions include caffeine reduction, weight loss and cessation of smoking. While more recent literature supports PFMT to treat urge and stress incontinence, its long-term efficacy remains uncertain.14,15 Pre-operative and early postoperative (immediately after catheter removal) PFMT have been found to significantly improve and hasten the recovery of continence rate,13–15 but there is limited evidence for preventive effects of pelvic floor rehabilitation. In some patients with co-existing urinary symptoms that are suggestive of mixed incontinence, the use of an antimuscarinic drug may be useful to eliminate potential detrusor overactivity.
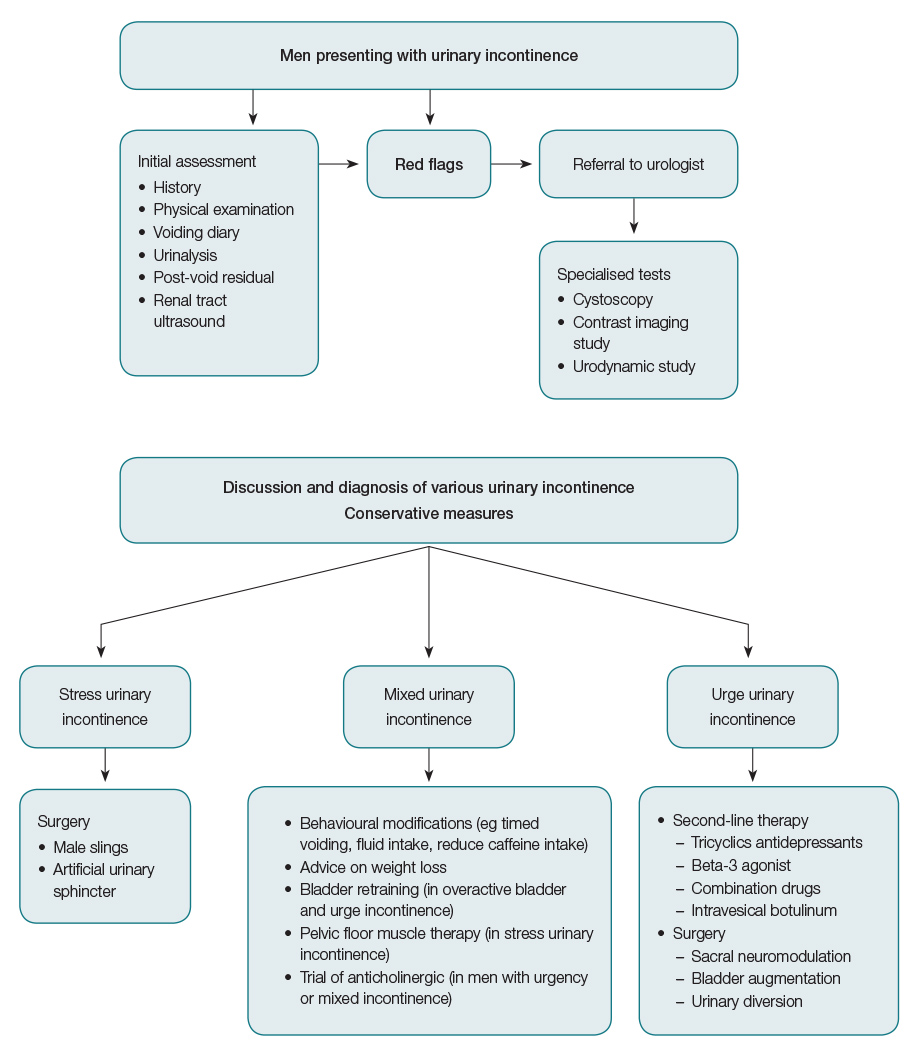
Figure 1. A practical algorithm in the management of adult male stress urinary incontinence and overactive bladder/urge urinary incontinence
In patients with persistent post-prostatectomy SUI, there is no approved medical therapy,8 and surgical treatment is recommended after failure of conservative measures.14,15 Published literature has found that the most significant improvement in SUI usually occurs during the first year, with minimal improvement usually seen beyond the second year postoperative.4,16 Urethral bulking agents are generally ineffective in men and are associated with the need for multiple injections, temporary benefit and a low cure rate.15,16 Minimally invasive male slings, such as transobturator AdVance (American Medical Systems, Minnetonka, MN, US) and Virtue (Coloplast, Minneapolis, MN, USA) slings, and adjustable slings with Argus (Promedon, Cordoba, Argentina) and ATOMS (AMI, GmbH, Feldkirch, Austria) are advocated in men with mild-to-moderate SUI without prior radiotherapy.15,16 There is no consensus concerning each specific sling system, and the implantation of the various male slings is based on surgeon expertise and patient preference. While adjustable male slings have a theoretical advantage over non-adjustable slings – because the sling can be revised easily to provide further urethral compression in the event of persistent and/or recurrent urinary incontinence without the need for another sling or salvage artificial urinary sphincter (AUS) surgery – recent literature has found no significant difference observed in the clinical outcomes with reported similar patient satisfaction rate.17
The current AUS, AMS 800 (American Medical Systems, Minnetonka, MN, US) is considered the standard of care in men with moderate-to-severe SUI and/or SUI that is associated with radiotherapy.18 Like any surgical device, male slings and AUS have their own merits and disadvantages, and complications include erosion, mechanical failure, infection and revision rates.18 While AUS remains the standard of care in men with significant stress urinary incontinence and/or radiation-induced urinary incontinence, with excellent long-term efficacy, durability and safety records, many men prefer a male sling to an AUS because it provides an instantaneous postoperative continence. Male slings are often viewed as minimally invasive and their use allows patients to void without mechanical manipulation of a urinary device.19
The treatment of OAB/UUI aims to increase bladder capacity, decrease bladder activity and contractility, and/or decrease sensory (afferent) input. Behavioural modifications with avoidance of stimulants (eg caffeine, smoking) and pharmacological therapy with anticholinergic agents are the mainstay of treatment for UUI.7–9,11–13,20 At present, there is no consensus on ‘best-in-class’ antimuscarinic drugs. The drug profiles, interaction and tolerability, and dosing schedule can differ between individuals and thus, the treatment should be individualised for each patient. The common adverse effects related to cholinergic profiles, namely dry mouth, blurred vision, tachycardia, constipation, impaired cognition and urinary retention, have often resulted in poor compliance and high discontinuation rates.7 When using antimuscarinic agents in patients at risk of worsening cognitive function, it may be appropriate to monitor changes during initiation and continuation of treatment. Salvaging non-responders to a drug by switching to another drug has been found to be effective in some studies and so switching between drugs (ie modality, subclass) is a reasonable approach for patients in whom the initial oral agent has failed.20,21 The beta-3 adrenoceptor agonist mirabegron represents a new class of drug and mechanism. It causes direct relaxation of detrusor muscle, inhibition of spontaneous contractile activity in the bladder and reductions in bladder afferent activity.22 While beta-3 adrenoceptor agonists are well tolerated and have no anticholinergic adverse effects (eg dry mouth), mirabegron has a potential effect on the beta receptors in the cardiovascular system, which can theoretically lead to an increase in cardiovascular events, particularly hypertension and headaches.
If patients become refractory to these initial conservative measures and/or are intolerant of the adverse effects, intravesical botulinum toxin and electrical stimulation using sacral neuromodulation are effective and considered third-line therapies in OAB.11,20 Botulinum toxin can be injected into the detrusor muscle cystoscopically, either in an office-based setting under local anaesthesia or the operating room. The long-term benefits of botulinum toxin appear to be sustainable, with an excellent safety profile.20,23 Sacral neuromodulation involves the insertion of an electrode into the S3 foramen, which provides nerve stimulation to the bladder and perineum.24 A temporary lead can be placed to provide stimulation for a short trial period (around seven to 14 days), after which a permanent lead and generator are surgically implanted. Sacral neuromodulation does have durable treatment effects, but there are adverse effects, including pain, lead migration, infection and the need for further procedures.25 Bladder reconstructive surgery with augmentation cystoplasty and detrusor myomectomy are infrequently performed these days, and urinary diversion is often reserved as the last option in severe intractable cases.20
Key points
- Adult male non-neurogenic urinary incontinence is a highly prevalent condition that has a significant impact on a patient’s quality of life and healthcare resources.
- Over the past decade, there have been considerable advances made in terms of our understanding of the various pathophysiological mechanisms and management strategies in male SUI and OAB/UUI.
- Management of men with urinary incontinence requires careful evaluation, with comprehensive history-taking, focused physical examination and basic investigations to identify the underlying pathology, and should be tailored to the individual patient.
- Management involves a frank discussion about the various treatment options and the patient’s expectations, before selecting the most appropriate treatment.
- Further research efforts for the development of novel drug therapies and minimally invasive surgery are necessary to meet the increasing prevalence and patients’ expectations.
Authors
Eric Chung MBBS, FRACS (Urology), Associate Professor of Surgery and Consultant Urological Surgeron, Androrology Centre, Brisbane, Qld; University of Queensland, Department of Urology, Princess Alexandra Hospital, Brisbane, Qld. ericchg@hotmail.com
Darren J Katz MBBS, FRACS (Urology), Urologist and Prosthetic Surgeon, Men’s Health Melbourne; and Urology Consultant, Western Health, Vic
Christopher Love MBBS, FRACS (Urology), Urological and Prosthetic Surgeon, Men’s Health Melbourne, Melbourne and Urology South, Moorabbin, Vic; Senior Urological Surgeon, Department of Urology, Monash Medical Centre, Clayton, Vic; Bayside Urology, Melbourne, Vic
Competing interests: None.
Provenance and peer review: Commissioned, externally and internally peer reviewed.