Chronic hepatitis B (CHB) affects an estimated 239,000 Australians. The majority of those affected were born overseas in high-prevalence or intermediate-prevalence countries.1 CHB can lead to liver cancer – specifically, hepatocellular carcinoma (HCC) – and important added risk factors include the presence of cirrhosis, age, region of birth, male gender, co-infection with hepatitis C, D or human immunodeficiency virus (HIV), and other active liver disease (eg alcohol-related injury, non-alcoholic steatohepatitis).2
Liver cancer is the fastest-increasing cause of cancer mortality in Australia.3 It is one of the few cancers with no improvement in five-year survival since 2009, and is projected to become the sixth most common cause of cancer mortality in Australia.4 Poor outcomes of liver cancer are occurring in an era when most other cancers (eg bowel, breast, cervical) are associated with improving survival because of a combination of early detection and improved treatment approaches.3
Reducing mortality from HCC due to CHB can be achieved with improvements in primary, secondary and tertiary prevention measures by preventing both new infections and the sequelae of existing infections.5 Prevention of HCC in people who are already living with CHB can be achieved through regular monitoring and commencement of antiviral therapy, when appropriate. Antiviral treatment is associated with a reversal of cirrhosis and a reduction of HCC risk by up to 75%.6 Tertiary prevention of the consequences of CHB is achieved by HCC surveillance, with early detection of tumours that are amenable to curative therapy contributing to reduced mortality.
In Australia, the number of patients requiring and participating in HCC surveillance is unknown. Significant gaps exist in the care cascade, which suggests that participation is likely to be poor. Forty per cent of people living with CHB remain undiagnosed and 80% are not receiving regular care.7 Late diagnosis of CHB prior to HCC or cirrhosis diagnosis occurs in an estimated one-third of people.8
Guidelines recommend HCC surveillance with liver ultrasonography, with or without alfa-fetoprotein measurement, every six-months.9 Liver ultrasonography has a sensitivity of 58–89% and specificity >90% for the detection of HCC.10 In Australia, recommendations on who should be enrolled in surveillance is in accordance with the American Association for the Study of Liver Disease (AASLD) guidelines, with a modification to include specific surveillance recommendations for Aboriginal and Torres Strait Islander peoples.11,12 The recommendations for HCC surveillance in people living with CHB are listed in Box 1.
Box 1. Current Australian recommendations for HCC surveillance for people living with CHB9
|
All people with cirrhosis
Those with a first-degree family history of HCC
Asian men aged >40 years, and Asian women aged >50 years
African people aged >20 years
Aboriginal or Torres Strait Islander people aged >50 years
|
General practice management of CHB is essential for improving enrolment in chronic disease care, including successful participation in HCC surveillance, and is cost-effective.13,14 However, HCC surveillance is difficult to implement in practice.15,16 Reported mortality benefits vary for surveillance-detected HCC, but the optimal interval of surveillance is not clear.15,17 Harms associated with HCC–surveillance, including the psychological impact and false-positive results, have not been well reported.18 A recent study reported that physical harm may occur in one-quarter of people in whom a lesion is identified; in most cases, harm was characterised as unnecessary imaging after a false-positive test.19 Optimal HCC surveillance requires clinicians to enrol appropriate patients, and for patients to attend for the scheduled ultrasonography.5 Factors associated with improved HCC surveillance include frequency of clinic visits (ie tertiary or primary care), specialist service involvement and higher socioeconomic status.15,16
In recognition of the need to support CHB care in general practice, the Integrated Hepatitis B Service (IHBS) was funded in Victoria from 2012 to 2016 to support general practices and link them with specialist units. The community health centre in this study is in western Melbourne, an area of high CHB prevalence, and servicing a large multicultural community. It became a partner of the IHBS after general practitioners (GPs) identified improving CHB management (including HCC surveillance) as a priority in their practice. IHBS nurses assisted the clinic by:
- conducting baseline audits
- advising GPs on guidelines-based care
- contacting patients lost to follow-up
- strengthening standard recall and reminder systems by posting radiology and pathology requests, and regular review and phone calls to individuals failing to attend appointments.
Return visits by the nurses were conducted every four months to follow up if patients had attended. The service used qualified interpreters on request.
The aim of this study was to describe adherence to HCC surveillance and monitoring in a general practice that received external support to improve CHB management, including HCC surveillance.
Methods
We retrospectively analysed the impact of systems support on HCC surveillance over the period of IHBS involvement. At baseline of the IHBS involvement an audit of CHB patients in the practice was conducted and data, including HCC surveillance eligibility, demographics, most recent ultrasound and HBV DNA, were recorded. Included in the group who received surveillance and were followed up by the IHBS were people who were contactable and agreed to have their care delivered by the practice. Eligibility for HCC surveillance was determined by current Australian recommendations. Final data collection 4.5 years after the initial audit included the date of all ultrasound examinations and HBV DNA viral load test results in the clinical record over the study period; a binary variable if the clinician ordered tests at least yearly; number of booked appointments not attended; and if pregnancy, significant illness or reasons for any periods of non-attendance occurred. New patients were included in the final audit with time under observation from first visit to the clinic. Participants who became eligible because of age during the study period were included from January of the year of eligibility. Consistency of care was measured from the last 10 visits prior to the end of the observation period as the proportion seen by a single provider.
Participation in HCC surveillance was defined as two consecutive ultrasound scans and at least one scan every two years. The optimal interval for surveillance is based on tumour doubling time, which is estimated to be six to eight months,10 and currently there is no clear international definition. In this study, adherence to HCC surveillance recommendations was classified as poor (average <1 scan every 14 months), suboptimal (average ≥1 but <2 scans every 14 months and good (average of ≥1 ultrasound every seven months). For each patient, the months to the last ultrasound from the first and second audit dates were calculated as a measure of improvement in surveillance because of increased focus on surveillance and IHBS involvement.
Data were collected in Microsoft Excel and analysed using Stata 11. Chi-squared and Fishers exact tests were used for difference of proportions. The study received human research ethics committee approval (Melbourne Health QA2013111).
Results
The baseline audit identified 80 patients who met the criteria for HCC surveillance; 37 were not enrolled in regular review because they received care elsewhere, were not contactable or declined follow-up (Figure 1). Sixty-seven patients received HCC surveillance in the community during the study period, representing 213 person years of follow-up. The number eligible for HCC surveillance in the clinic increased from 43 to 63 individuals and the proportion of patients being managed in hospital changed from 25% to 15%(P = 0.055).
The median age of patients undergoing surveillance was 37.6 years (interquartile range [IQR]: 28.6–50.2); 43 (64%) were born in a country in the sub-Saharan African region; and five (8%) had been diagnosed with cirrhosis. The overall participation rate was 75%; 13% underwent surveillance less frequently than every two years; and eight (12%) participants had no ultrasound examinations over the study period despite tests being ordered by the clinician at least yearly, with postal and telephone reminders. Clinicians ordered ultrasonography at least every 12 months for 60 (90%) patients. Failure to attend appointments occurred for 51 (76%) patients, with half of all patients having more than five missed appointments over the 4.5-year period. Characteristics associated with adherence categories are shown in Table 1.
Table 1. Characteristics by adherence to ultrasound surveillance and P value for difference of proportions
|
Characteristic
|
All
(n = 67)
|
Good adherence
(n = 18)
|
Suboptimal adherence
(n = 29)
|
Poor adherence
(n = 20)
|
P value
|
|---|
|
Sex male (%)
|
43 (64.2)
|
10 (23.3)
|
17 (39.5)
|
16 (37.2)
|
0.22
|
|
Age <35 years
|
29 (43.3)
|
5 (17.2)
|
13 (44.8)
|
11 (37.9)
|
0.27
|
|
Reason for surveillance
|
|
Region of birth
|
57 (85.1)
|
12 (21.1)
|
26 (45.6)
|
13 (33.3)
|
0.08
|
|
Cirrhosis
|
5 (7.5)
|
4 (80.0)
|
1 (20.0)
|
0
|
|
|
Family history
|
5 (7.5)
|
2 (40.0)
|
2 (40.0)
|
1 (20.0)
|
|
|
On treatment
|
16 (24.6)
|
8 (50.0)
|
7 (43.7)
|
1 (6.3)
|
0.03
|
|
Provider consistent*
|
41 (61.2)
|
13 (31.7)
|
20 (48.8)
|
8 (19.5)
|
0.08
|
|
Managed <2 years
|
14 (20.9)
|
9 (64.3)
|
2 (14.3)
|
3 (21.4)
|
<0.01
|
|
Viral load ≥every 14 months
|
39 (58.2)
|
16 (41.0)
|
19 (48.7)
|
4 (10.3)
|
<0.01
|
|
*Provider consistent – same clinician for more than seven of 10 visits
|
A greater proportion of patients received an ultrasound examination in the seven months prior to the final audit, compared with the baseline audit (56% versus 10%; P <0.001). Four patients ceased surveillance at the clinic as three had transferred their care and one became HBsAg-negative and did not require further surveillance. One-quarter (17 participants) of patients who did not attend care had reasons recorded in their patient records, which included travel overseas, pregnancy and other significant health issues. Three potentially suspicious lesions that required further investigation were identified over the study period, but no liver cancers were identified. Thirty-four (51%) participants had a maximal interval between screening tests of >14 months during the observation period. As any measure of adherence missed variation over time, a visual representation of adherence showing individual-level data over the observed period is shown in Figure 2.
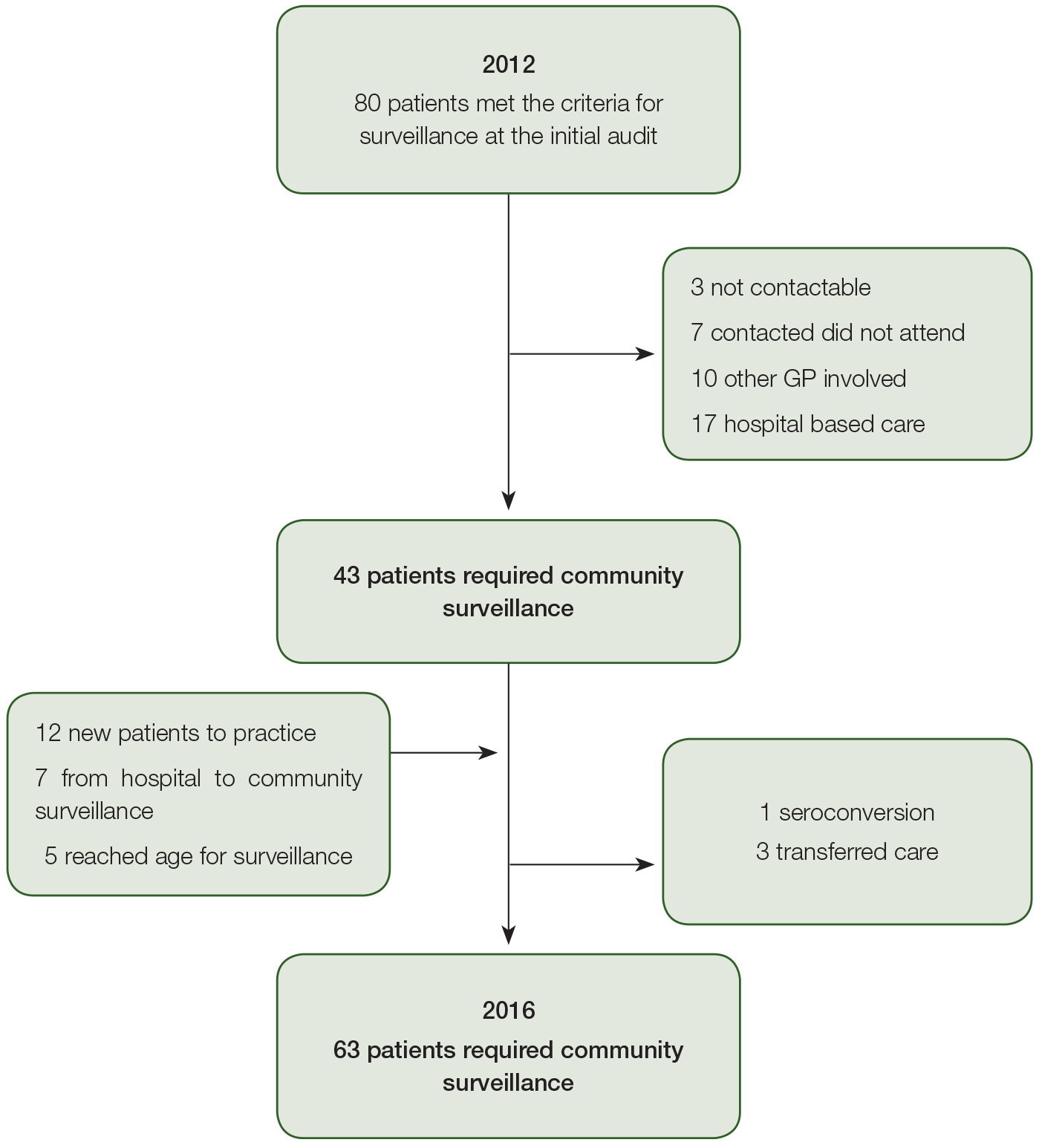
Figure 1. Flow of patients requiring liver cancer surveillance in and out of a community clinic 2012–16
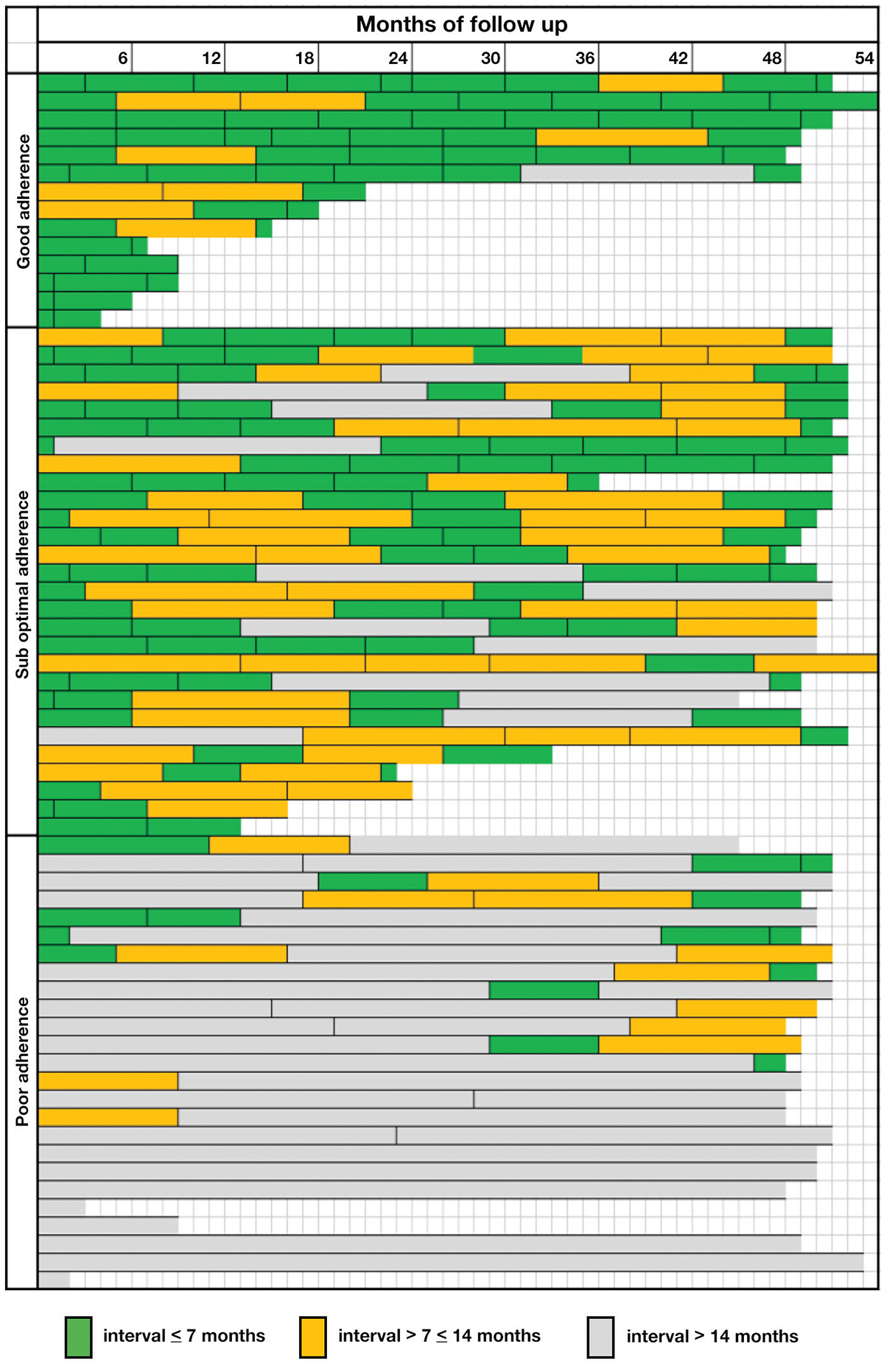
Figure 2. Visual representation showing individual-level data, intervals of ultrasound surveillance and categories of adherence
Discussion
Delivering guideline-based HCC surveillance in general practice presents substantial challenges. In the supported setting described in this study, participation in HCC surveillance was higher than in other clinical cohorts,5,15,16 and participation improved over the study period. However, optimal adherence to recommended surveillance intervals was only achieved in a quarter of patients. This is despite external clinician support, provider consistency (70% of clinic visits with single provider in two-thirds of patients), regular ordering of tests, and regular reminder letters and telephone contact. Patients were more likely to adhere to optimal surveillance intervals if they were on antiviral treatment, more recently diagnosed and having regular viral load tests.
While participants’ adherence was defined in this analysis as the number of ultrasound examinatons over the study period, the patterns of adherence varied among individuals and over time. In this cohort, patients who were lost to follow-up for a period could re-engage, reflecting real-world data. Other factors, such as pregnancy, travel and other significant illness, also affected adherence to surveillance. Half of the patients who had regular viral load tests had suboptimal or poor interval surveillance. This suggests that barriers to testing with ultrasongraphy may differ from barriers for blood tests, which were available at the clinic and did not require a separate booking or further attendance.
Limitations of this study include the size and specific clinic population. Individuals might have received care from other clinics, where other practitioners may also have order ultrasonography for surveillance. The impact of this would be to underestimate surveillance frequency. The findings are also unlikely to be generalisable to other community-based settings, particularly given that the clinic was staffed by GPs with an interest in hepatitis B (and refugee health), and received support from an external expert tertiary service. The study period was characterised by the partnership between IHBS and clinic, but this study does not seek to formally evaluate the program; rather, it describes the impact of systems support on HCC surveillance.
This study shows that intensive and supported systems improvement, while likely to improve HCC surveillance frequency, does not completely address complex issues involved in long-term, frequent cancer surveillance of people who (for the vast majority) have no symptoms related to their condition, and many of whom (particularly those born in Africa) are young. Family, employment, accommodation, other health priorities, health literacy, and other factors may all interfere with the ability to book and attend regular ultrasonography appointments in addition to clinic appointments and onsite pathology tests.
Optimal adherence to liver cancer surveillance is difficult to achieve. In Australia, this surveillance is not supported by external registries, media campaigns or appropriate educational material to promote surveillance among affected communities where people may have low health literacy. CHB care (including HCC surveillance) is a current national strategic direction, and needs to occur in general practice and tertiary centres to reach the many people eligible and not receiving care.14 Participation in more established cancer-screening programs (ie bowel, breast, cervical) is lower in overseas-born Australians because of reduced healthcare access, lower health literacy, and other cultural perceptions and understanding of cancer and cancer prevention.20,21 There are few studies of the barriers to, and knowledge of, HCC surveillance in affected communities.22 Overseas experience recall systems have had mixed success in improving adherence.23
There are many outstanding questions in HCC surveillance, including the effects of variability in the quality of scans, expertise of reporting radiologists and different surveillance intervals, and at what time interval suboptimal surveillance frequency confers a mortality benefit.17,18,24 These outstanding questions are complicated by a lack of consistency in definitions of adherence and reporting of outcomes.18 There is policy discussion about the value of additional systems, including registries to enhance HCC surveillance. In New Zealand, Korea and Japan, reduction in mortality for HCC has been observed in population-based registries.25
In conclusion, this study has demonstrated the challenges in enhancing HCC surveillance in general practice. Future interventions will require an understanding of the target population and barriers they face to achieving optimal surveillance, including knowledge and risk perception. Systems improvement alone is unlikely to achieve good adherence to current guidelines for HCC surveillance.
Authors
Nicole Allard MBBS, FRACGP, MPH, General Practitioner, cohealth, Footscray, Vic; Medical Epidemiologist, WHO Collaborating Centre for Viral Hepatitis, Peter Doherty Institute for Infection and Immunity, University of Melbourne, Parkville, Vic; PhD candidate, University of Melbourne, Parkville, Vic. nicole.allard@mh.org.au
Tracey Cabrie MPH, GradDipIntHlth, BNurs, Victorian Infectious Diseases Service, Melbourne Health, Royal Melbourne Hospital, Parkville, Vic
Emily Wheeler MPH, RN, Victorian Infectious Diseases Service, Melbourne Health, Royal Melbourne Hospital, Parkville, Vic
Jacqui Richmond PhD, MPH, RN, Research Fellow, Australian Research Centre for Health Sex and Society, La Trobe University, Melbourne, Vic
Jennifer MacLachlan BSc, MPH, Epidemiologist, WHO Collaborating Centre for Viral Hepatitis, Peter Doherty Institute for Infection and Immunity, University of Melbourne, Parkville, Vic
Jon Emery MBBCh, MA, MRCGP, FRACGP, DPhil, Herman Professor of Primary Care Cancer Research, University of Melbourne, Parkville, Vic; General Practitioner, cohealth, Footscray, Vic
John Furler MBBS, MRCGP, FRACGP, GradDipPubHlth, PhD, Department of General Practice, Faculty of Medicine, Dentistry and Health Science, University of Melbourne, Parkville, Vic; General Practitioner, cohealth, Footscray, Vic
Benjamin Cowie MBBS, PhD, FRACP, Director, WHO Collaborating Centre for Viral Hepatitis, Peter Doherty Institute for Infection and Immunity, University of Melbourne, Parkville, Vic; Physician, Victorian Infectious Diseases Service, Royal Melbourne Hospital, Parkville, Vic
Competing interests: None
Provenance and peer review: Not commissioned, externally peer reviewed
Acknowledgement
Nicole Allard was supported by an Australian Postgraduate Award as part of her PhD studies.