Pulmonary embolism, first described by Virchow in the 1800s, was often a terminal event. A 1960 trial on the efficacy of heparin in pulmonary embolism found a mortality rate of 17%,1 and noted that ‘pulmonary embolism was rarely diagnosed before death’. The Therapeutic Guidelines2 introduces pulmonary embolism as ‘frequently underdiagnosed’, with ‘a high mortality if untreated; continued suspicion of and urgent therapy for pulmonary embolism is therefore required’. Assertions such as this have led to a hyper-vigilance about the diagnosis of pulmonary embolism.
The Centers for Disease Control and Prevention estimate there are 60,000–100,000 deaths per annum from pulmonary embolism in the US.3 Mortality data in Australia and the UK do not support such figures. In 2015, there were 340 deaths (0.2% of all deaths) from pulmonary embolism in Australia.4 In the UK, there were 2300 deaths from pulmonary embolism in 2012,5 which accounted for 0.4% of all deaths.6
Pulmonary emboli large enough to cause haemodynamic compromise are a major source of morbidity and mortality. However, modern tests, especially multidetector row computed tomography pulmonary angiography (CTPA), have changed the nature of pulmonary embolism as a clinical entity. From 1998 to 2006, the rate of pulmonary embolism detection in the US nearly doubled without any change in mortality.7
There is a growing realisation that although the presence of a large pulmonary embolus is a serious and potentially fatal event, we are now, with better technology, able to detect pulmonary emboli that were previously missed but not necessarily clinically relevant.7,8
The challenge with cases of potential pulmonary embolism is to weigh up the relevance of diagnosis and the benefits of treatment against the harms of not only the treatment but also the investigation.
Clinical presentation
Acute onset of dyspnoea and chest pain, especially pleuritic in nature, generally leads to consideration of pulmonary embolism as a possible diagnosis. Other symptoms, such as cough and haemoptysis, concurrent symptoms of deep venous thrombosis (DVT), and signs of tachypnoea, tachycardia and hypoxia, may also be present. However, chest pain and dyspnoea are common symptoms in general practice and emergency departments, and the vast majority of these patients will not have pulmonary embolism.
Risk stratification
History
There are numerous risk factors for pulmonary embolism, some of which are included in Box 1. These, along with other features on presentation, help determine the clinical impression or gestalt of the presence or absence of pulmonary embolism.
Historically, pulmonary embolism had high morbidity and mortality rates. However, the modern ability to detect even the smallest pulmonary emboli means we are currently detecting ‘disease’ that used to be considered part of the normal function of the lung – to filter small clots. The mortality of otherwise healthy individuals, in the outpatient setting, with proven pulmonary embolism and normal physiology approaches 0%.8 This is lower than the mortality associated with diagnosing and treating pulmonary embolism in this subgroup. Numerous studies suggest that small pulmonary emboli are transient and normal,8 and that the diagnosis of pulmonary embolism in the modern era should not ‘chill the marrow of clinicians’.8
Without question, pulmonary embolism can be a devastating and fatal diagnosis; however, the ability to detect pulmonary emboli of all sizes leaves clinicians with a conundrum. If we seek to diagnose every pulmonary embolus, even ones that evidence suggests are ‘normal’, then at some point along the spectrum, we will start to cause more harm than good. The difficulty is knowing where that point is.
Box 1. Risk factors for pulmonary embolism
|
Surgery – major joint surgery, lower limb surgery, abdominal or pelvic surgery for cancer, major gastrointestinal tract surgery, multi-trauma, spinal cord injury with paresis
|
|
Acute and chronic medical illness – chronic heart failure, myocardial infarction, inflammatory bowel disease, active rheumatic disease, nephrotic syndrome, acute respiratory failure, chronic lung disease
|
|
Malignancy-related factors – active malignancy, myeloproliferative neoplasms, cancer treatment
|
|
Hormonal-related factors – pregnancy or early postpartum, oral contraceptive pill, hormone replacement therapy
|
|
Known thrombophilia
|
|
Other – body mass index >30 kg/m2, venous stasis/varicose veins, past history of deep venous thrombosis or pulmonary embolism, prolonged immobilisation/travel
|
The current solution to this problem is to risk-stratify patients with suspected pulmonary embolism, and to use validated risk stratification tools to guide investigation. There are numerous clinical decision rules, including the Wells score (Table 1), modified Wells score, simplified Wells score, revised Geneva score, Charlotte rule and Pulmonary Embolism Rule-out Criteria (PERC) rule. The Wells score and PERC rule are the most validated tools of these studies,9 are simple to use, and can be incorporated into the assessment of patients with suspected pulmonary embolism.
Table 1. Wells criteria
|
Clinical feature
|
Wells score
|
|---|
|
Clinical signs and symptoms of DVT
|
3
|
|
Pulmonary embolism most likely diagnosis
|
3
|
|
Heart rate >100 beats per minute
|
1.5
|
|
Immobilisation at least three days or surgery within past four weeks
|
1.5
|
|
Previous DVT or pulmonary embolism
|
1.5
|
|
Haemoptysis
|
1
|
|
Malignancy treatment within six months or palliative
|
1
|
|
DVT, deep venous thrombosis
A Well’s score >4 warrants imaging
|
Rules such as the Wells score incorporate clinical impression, to a degree, into their scoring system. A 2011 systematic review found that clinical decision rules such as Wells score were more specific and just as sensitive as clinical impression.10
The greater the specificity of a test, the better it is at ruling the condition in (a positive result is likely to be a true positive); the greater the sensitivity, the better the test is at ruling the disease out (a negative result is likely to be a true negative). This allows more rational use of investigations, and the benefits of this are a reduction in exposure to ionising radiation (especially breast tissue in women of child-bearing age), decreased risk of reactions to intravenous contrast, and a reduction in healthcare costs to the patient and society.
Wells score comprises a set of mostly objective criteria designed to determine a pre-test probability for pulmonary embolism. Calculators for Wells criteria and the PERC rule are available online (www.mdcalc.com). Wells score can only be applied if symptoms have been present for <30 days, and is not validated for use if:9
- upper limb DVT is suspected as a source of pulmonary embolism
- the patient has been on anticoagulants for >72 hours
- the patient has been asymptomatic for 72 hours prior to presentation
- the patient is pregnant.
If clinical signs and symptoms of DVT are the clinical features that make the Wells or simplified Wells score sufficiently positive to warrant imaging then, intuitively, a negative venous ultrasound scan could make the patient low risk and potentially reduce the need for CTPA or ventilation/perfusion (V/Q) scanning. There is no specific evidence in the literature for this approach, but one recent study that combined lung and venous ultrasound scanning with Wells criteria led to a 23% reduction in CTPA ordering.11
A Wells score ≤4 makes pulmonary embolism unlikely, but does not fully exclude it. Tests such as D-dimer can add greater certainty to excluding pulmonary embolism as a diagnosis. In low-risk patients, a positive D-dimer is more likely to be a false positive than a true positive.9 Observations such as this led to the development and validation of the PERC rule.12,13 If the PTP is low using the Wells score then the PERC rule (Box 2) can be applied. If the answer to all of the criteria in Box 1 is ‘Yes’, then the PERC rule is negative. No further testing is required and pulmonary embolism is safely excluded.
Box 2. PERC rule
|
Aged <50 years
Pulse <100 beats per minute
SaO2 ≥95%
No haemoptysis
No oestrogen use
No surgery or trauma requiring hospitalisation within four weeks
No prior venous thromboembolism
No unilateral leg swelling
|
The PERC rule validation study13 included patients who presented with a primary complaint of shortness of breath or chest pain, and it is reasonable to use it for either of these symptoms. The PERC rule has not been validated for people with:
- active cancer, thrombophilia or a strong family history of thrombophilia
- transient tachycardia or beta-blocker use that may mask tachycardia
- leg amputations
- morbid obesity (leg swelling not easily determined)
- baseline hypoxaemia when oximetry reading <95% is longstanding.
If the patient’s PERC score is >0, then an enzyme-linked immunosorbent assay (ELISA)-type D-dimer is recommended. If this is negative, pulmonary embolism is ruled out and no further investigation is required; if positive, then imaging is recommended. The approach to the investigation above is summarised in Figure 1.
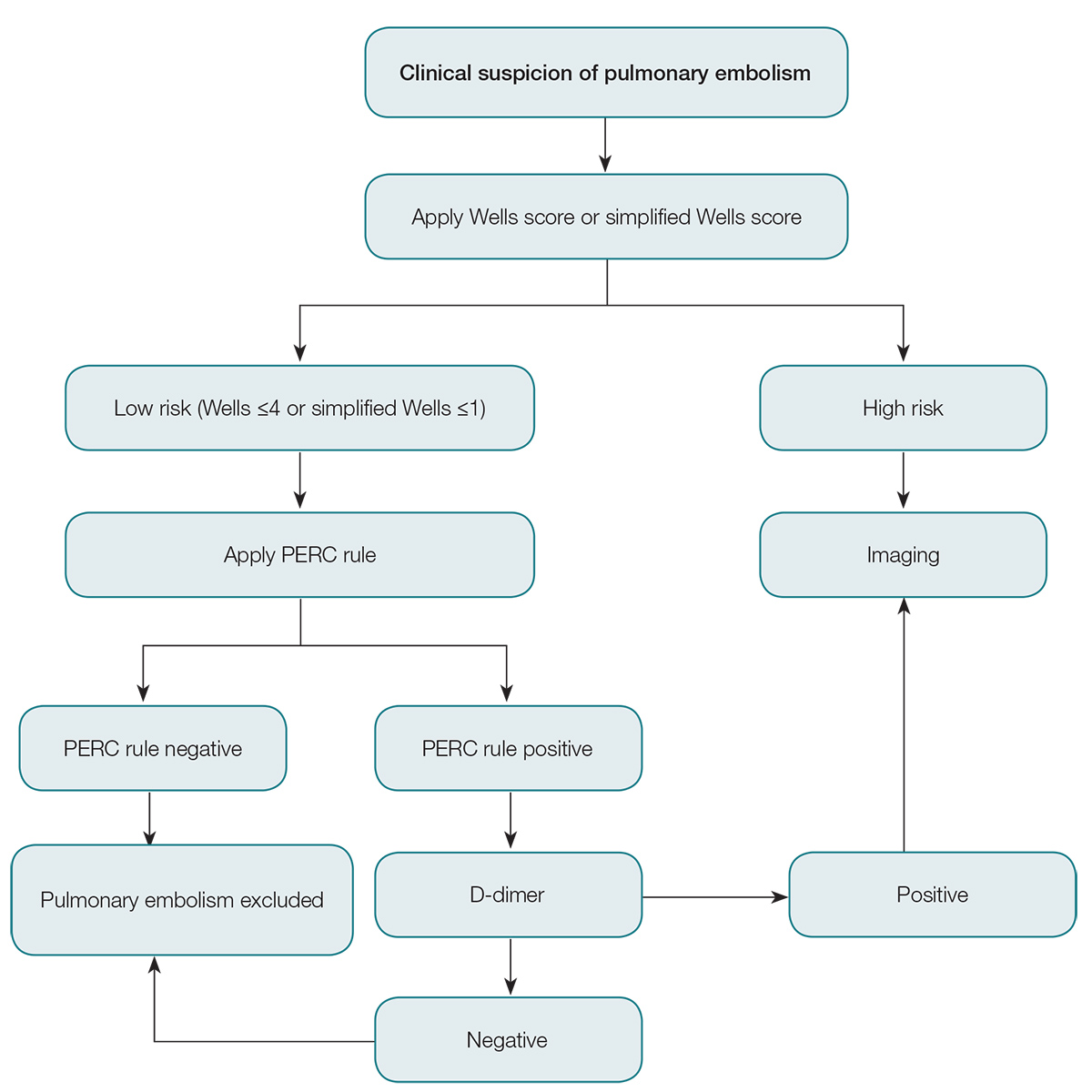
Figure 1. Approach to investigation of pulmonary embolism
PERC, Pulmonary Embolism Rule-out Criteria
Imaging
If imaging is required, this should be performed as soon as possible and urgently (via the emergency department) if there are significant cardiac or respiratory signs, such as tachypnoea, hypotension, tachycardia or hypoxia.9 If deemed less urgent, or if access to imaging is limited (eg remote location, weekends), it is reasonable to commence low–molecular weight heparin (LMWH) and arrange imaging for the next available day.14
Chest X-ray remains useful in determining alternative diagnoses (eg pneumothorax, pneumonia) in appropriate clinical cases. Definitive diagnosis will require CTPA or V/Q scanning. CTPA is the most commonly used modality, but V/Q scanning should be considered the modality of choice in pregnancy if the scanning device is readily available.9
For severely compromised patients, bedside echocardiography findings of right ventricular dilation, right ventricular hypokinesis and high right atrial pressures may confirm the presence of massive pulmonary emboli.
Imaging considerations in pregnancy
Radiation risk in pregnancy relates to maternal and fetal risk. The radiation dose to the breast is much higher with CTPA than with V/Q scanning, and CTPA has a significantly higher maternal risk.9 Radiation risk to the fetus is low and comparable with both CTPA and V/Q scanning.9 Patients who are pregnant are generally younger and have fewer comorbidities than non-pregnant patients with suspected pulmonary embolism. V/Q scanning, especially in the presence of a normal chest X-ray, is a more reliable test in pregnancy than in the non-pregnant population.15 Low-dose perfusion-only scanning minimises the radiation dose to the fetus and is safe; however, if concerns persist, exposure to radiation can be further reduced by using a urinary catheter, which removes isotope from the bladder more quickly.16
Treatment
Anticoagulation is the mainstay of treatment for pulmonary embolism. Massive pulmonary embolism may warrant thrombolytic therapy. One controversy is the benefit or otherwise of treating subsegmental pulmonary embolism (SSPE).
Thrombolysis
For severely compromised patients, the American College of Chest Physicians (ACCP) recommend systemic thrombolysis if systolic blood pressure is <90 mmHg.17 The American Heart Association18 also states that ‘fibrinolysis is reasonable for patients with massive acute pulmonary embolism and acceptable risk of bleeding complications’. This includes patients with a systolic blood pressure <90 mmHg or bradycardia <40 beats/minute, and that ‘fibrinolysis may be considered for … submassive acute pulmonary embolism (with) … hemodynamic instability, worsening respiratory insufficiency, severe right ventricular dysfunction, or major myocardial necrosis and low risk of bleeding complications’. Patients with massive pulmonary embolism will generally be managed in the hospital setting, and the Therapeutic Guidelines recommend heparin infusion and alteplase.2
Anticoagulation
LMWH reduces complications and thrombus size, compared with unfractionated heparin, for the initial treatment of VTE without altering mortality.19 The Therapeutic Guidelines2 recommendations for the treatment of acute pulmonary embolism are dalteparin 200 U/kg, up to 18,000 U daily or 100 U/kg, up to 9000 U twice daily; or enoxaparin 1.5 mg/kg daily or 1 mg/kg twice daily.Twice-daily dosing is preferred if the risk of bleeding or thrombus extension is high (eg older age, obesity, malignancy). If creatinine clearance is <30 mL/min, dose adjustment is required.
Warfarin should be commenced and the INR maintained at 2–3. Rivaroxaban is currently approved and subsidised for use in pulmonary embolism in Australia, and can be used as an alternative to LMWH and warfarin in the treatment of pulmonary embolism.20,21 High-risk patients, such as those with antiphospholipid syndrome and recurrent unprovoked pulmonary embolism, were excluded from the clinical trials.20 The dose of rivaroxaban is 15 mg twice daily for three weeks, then 20 mg daily. Treatment duration is six months, but may be three months in the presence of a transient major risk factor, or indefinite if there are ongoing major risk factors (eg cancer, recurrent unprovoked pulmonary embolism).
Subsegmental pulmonary embolism
The ACCP17 recommends that clinical surveillance is preferred to anticoagulation for patients with SSPE (no involvement of proximal pulmonary arteries) and no proximal DVT with low risk for recurrent VTE. The ACCP adds that ultrasound scanning of the deep veins in both legs should be performed to exclude proximal DVT, and that clinical surveillance may be supplemented by serial ultrasound scanning.
In a retrospective review, Donato et al22 found that patients with SSPE had favourable outcomes at three months without anticoagulation, and may do better without treatment, although there were only 22 patients in the no-treatment group, none of whom had recurrent pulmonary embolism.
Recommendations to not treat SSPE are weak as there are no randomised trials on the safety of anticoagulation versus no treatment in this subgroup;23 GPs may want to seek advice from specialist colleagues for this group.
Author
Steven Doherty PhD, MBBS, FACEM, Critical Care VMO Tamworth and Armidale Rural Referral Hospitals, Adjunct Professor University of New England, Armidale, NSW. steven.doherty@hnehealth.nsw.gov.au
Competing interests: Dr Doherty reports personal fees from Pfizer India outside the submitted work.
Provenance and peer review: Commissioned, externally peer reviewed.