Case
A university student, 21 years of age, presents with concerns regarding a 12-month history of amenorrhoea. She is otherwise well and has no significant medical or surgical history or recent weight loss.
The patient recalls menarche at 16 years of age, but her menses have been erratic, with only one day of light menstrual bleeding every three to four months. She has never been sexually active. She denies a history of hirsutism, vasomotor symptoms and family history of early menopause. She is a non‑smoker and non-drinker.
Clinical examination is essentially normal, including a height of 164 cm, body mass index (BMI) of 24 kg/m2 and Tanner stage IV breast and pubic hair development. She is normotensive. Biochemical investigations show an elevated follicle-stimulating hormone (FSH) level of 56 IU and a low sensitive oestradiol of <18 pmol/L, confirmed on at least two occasions over subsequent reviews. Thyroid function is normal.
The combination of prolonged amenorrhoea (>4 months) with an FSH in the menopausal range on two occasions at least four weeks apart is consistent with a diagnosis of premature ovarian insufficiency (POI).
Presentations of menstrual abnormalities are common in primary care, and POI is often an under-recognised cause. POI, defined as the ‘development of amenorrhea due to loss of ovarian function before the age of 40’,1 encompasses premature menopause (menopause before 40 years of age)2 and primary amenorrhoea (absence of spontaneous menarche). POI can be associated with a fluctuating and unpredictable course, with a small possibility that ovarian function may spontaneously resume. The term POI is increasingly preferred to ‘primary/premature ovarian failure/menopause’ as it more accurately reflects the variability in the clinical picture, and removes the negative connotations associated with the word ‘failure’.1
Depletion of ovarian follicles with POI leads to a decline in oestradiol, anti-Müllerian hormone and inhibin B levels, and a rise in pituitary gonadotrophins.3 Women with POI typically present in the primary care setting with primary or secondary amenorrhoea and infertility, and may have symptoms of oestrogen deficiency. Early diagnosis is important as women are at risk of morbidity, such as infertility, osteoporosis, accelerated cardiovascular disease (CVD) and neurocognitive disorders, and increased mortality.1 Delayed diagnosis also misses the opportunity for timely institution of oestrogen therapy. General practitioners (GPs) play a vital role in the evaluation and initial management of women with POI, and also in monitoring for long-term consequences.
Aetiology
The aetiology of POI is diverse, and it can occur spontaneously or be secondary to medical therapies. It is estimated that spontaneous POI affects 1% of the female population.4 However, evidence suggests that the prevalence of POI due to medical therapies (eg chemotherapy, ionising radiotherapy, bilateral oophorectomy) may be higher.5 Common causes of POI and associated conditions are listed in Box 1.
Spontaneous POI can be associated with chromosomal and genetic defects, environmental factors, autoimmune diseases (most commonly adrenal and thyroid disease) and various infections, but is idiopathic in the majority of cases.1
The most clinically important autoimmune conditions associated with POI are autoimmune adrenal disease (Addison’s disease) and thyroid autoimmunity. Addison’s disease can precede the diagnosis of POI or occur many years after its diagnosis. Adrenal insufficiency has been reported to affect 50% of women with spontaneous POI who have positive anti-adrenal antibodies and can occur 8–14 years following the diagnosis of POI.6 Thyroid autoimmunity in idiopathic POI typically manifests as hypothyroidism and occurs in up to 27% of cases.7 An elevated level of thyroid peroxidase auto-antibody should be followed with annual screening of thyroid function.
A family history of POI and cigarette smoking are well recognised risk factors for the development of POI,3 as is bilateral ovarian surgery for endometriomas, with a reported 2.3% of women developing POI.8 Nulliparity, hysterectomy, illicit drug use and adverse life events are also potentially implicated.9 Importantly, the combined oral contraceptive pill (COCP), fertility drugs and prior hormone replacement therapy (HRT) do not cause POI, but cessation of these therapies may unmask undiagnosed POI.
Box 1. Premature ovarian insufficiency – Causes and associations
|
Spontaneous POI
Idiopathic – most common cause of spontaneous POI
Genetic causes (10% of POI) –
- Turner syndrome (45XO) – most common genetic cause
- Fragile X pre-mutation (FMR1)
- Other: FOXL2, NR5A1, BMP15, FSHR, Gs alpha genes
Autoimmune associations (20% of POI) –
- Addison’s disease
- Autoimmune polyendocrine syndromes 1 and 2
- Autoimmune hypothyroidism
- Other autoimmune conditions: coeliac disease, type 1 diabetes mellitus, myasthenia gravis, systemic lupus erythematous, thrombocytopenic purpura, vitiligo, alopecia, pernicious anaemia, rheumatoid arthritis, Crohn’s disease, Sjogren’s syndrome, primary biliary cirrhosis
Inborn error of metabolism (rare causes of POI) –
Infectious causes
- Mumps oophoritis
- Associated infectious conditions: human immunodeficiency virus (HIV), tuberculosis, malaria, shigellosis, Herpes zoster, cytomegalovirus
Environmental associations
- Smoking – associated with earlier onset of menopause
Iatrogenic POI
Chemotherapy – particularly alkylating agents and dependent on cumulative dose
Radiotherapy – dependent on cumulative dose and field of exposure
Bilateral oophorectomy
Other pelvic surgery has been associated with early age of menopause and/or reduced ovarian reserve –
- Single oophorectomy, hysterectomy, uterine artery embolization, bilateral ovarian surgery for cysts or endometriosis
|
Clinical presentation
The clinical presentation of POI can be variable, but the most common presenting symptom is menstrual disturbance, particularly oligomenorrhoea or amenorrhoea. POI can have a significant negative impact on physical and emotional wellbeing, including menopausal symptoms, infertility and increased risk of long-term consequences (Figure 1).10
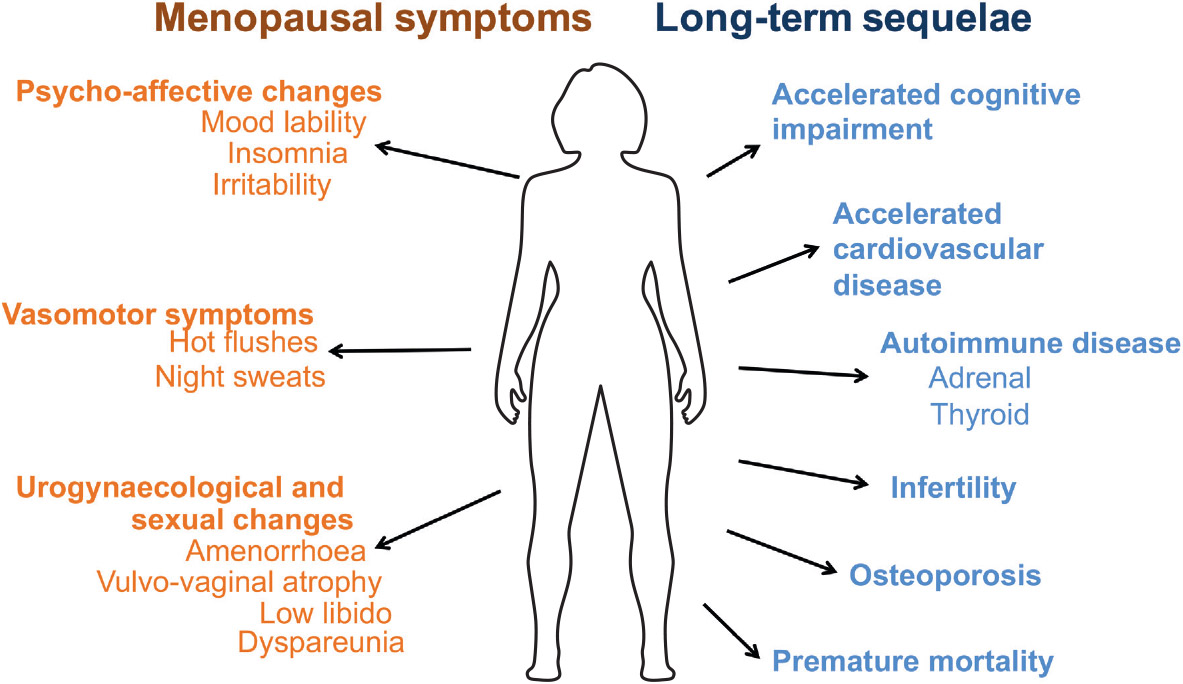
Figure 1. Consequences of premature ovarian insufficiency
Menopausal symptoms (eg hot flushes and urogynaecological and sexual changes) may be more severe in women with premature menopause, compared with natural menopause, whereas women with primary amenorrhoea are unlikely to experience menopausal symptoms.1
Infertility is a key feature of POI given the loss of ovarian reserve. In women with spontaneous POI, approximately 5% can spontaneously ovulate and conceive,11 and oocyte or embryo donations are the only reliable methods of achieving pregnancy.1 Fitness for pregnancy requires assessment in the preconception period as some women with POI may have high obstetric risk, such as women with Turner syndrome.12
Women with POI may present with adverse psychosocial symptoms, and have been found to have higher levels of depression and anxiety, a more negative body image, decreased sexual function and reduced confidence, compared with premenopausal controls.13 They may express grief at the loss of femininity and fertility, fear of long-term health consequences related to POI, and concerns regarding the effects on the relationship with their partner.13
POI may be diagnosed as a consequence of a comorbid condition, and the clinical presentation may be related to the underlying cause, such as thyroid or adrenal autoimmune conditions or cancer-related medical therapies (Box 1).
Diagnosis
POI should be considered in any woman aged <40 years presenting with a history of menstrual disturbance, especially oligomenorrhoea or amenorrhoea, regardless of whether they have symptoms of oestrogen deficiency. Further, girls who have not undergone menarche by 15 years of age should be investigated for primary amenorrhoea, as 95–98% of adolescents will have their first menses by this age.14 Diagnosis of POI can be difficult because of the variable and fluctuating presentation, reflecting fluctuating ovarian activity. At times, difficulty interpreting hormone results can hinder diagnosis. In a study of Australian women with POI, diagnosis took longer than two years in 23% of women, with at least two clinicians consulted on average.13 Where bilateral oophorectomy has occurred, diagnosis of POI is obvious.
Diagnosis of POI requires FSH levels in the menopausal range on two occasions at least four to six weeks apart in a woman aged <40 years after more than four months of amenorrhoea or menstrual irregularity, and after secondary causes of amenorrhoea have been excluded (Figure 2). According to International Menopause Society (IMS) guidelines,15 an FSH level >40 IU is used to define the menopausal range, while recent guidelines from the European Society of Human Reproduction and Embryology (ESHRE)1 recommend an FSH level >25 IU. It is important that women are not taking hormonal contraceptives or HRT, to ensure accurate interpretation of the hormone levels. These agents must be withdrawn for at least six weeks prior to hormone measurements. The routine use of anti-Müllerian hormone levels in the diagnosis of POI is not currently recommended as its accuracy is not validated in this setting.1
Further evaluation
Following diagnosis, the aetiology of POI (Figure 2) and long-term consequences (Figure 3) should be evaluated, and specialist referral may be necessary. In the absence of clear iatrogenic causes of POI, investigations should include:1
- karyotype – to exclude Turner syndrome and other chromosomal abnormalities
- FMR1 premutation testing (if karyotype normal) – Fragile X premutation carrier status
- autoimmune screen – including anti-thyroid peroxidase antibody, anti-adrenal antibody and coeliac serology.
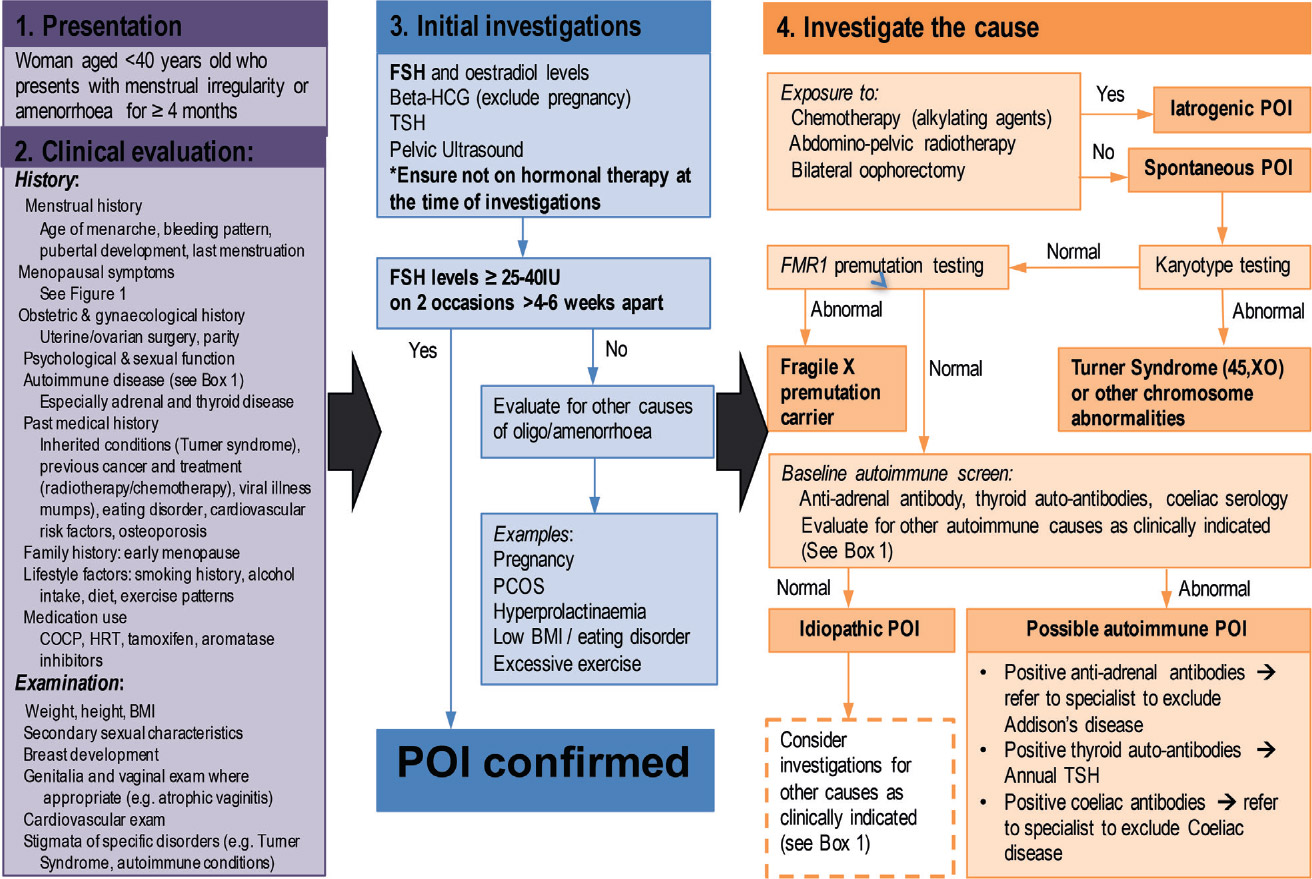
Figure 2. Premature ovarian insufficiency diagnostic algorithm
Abbreviations: COCP = combined oral contraceptive pill; HRT = hormone replacement therapy; BMI = body mass index;
FSH = follicular stimulating hormone; TSH = thyroid stimulating hormone; PCOS = polycystic ovarian syndrome
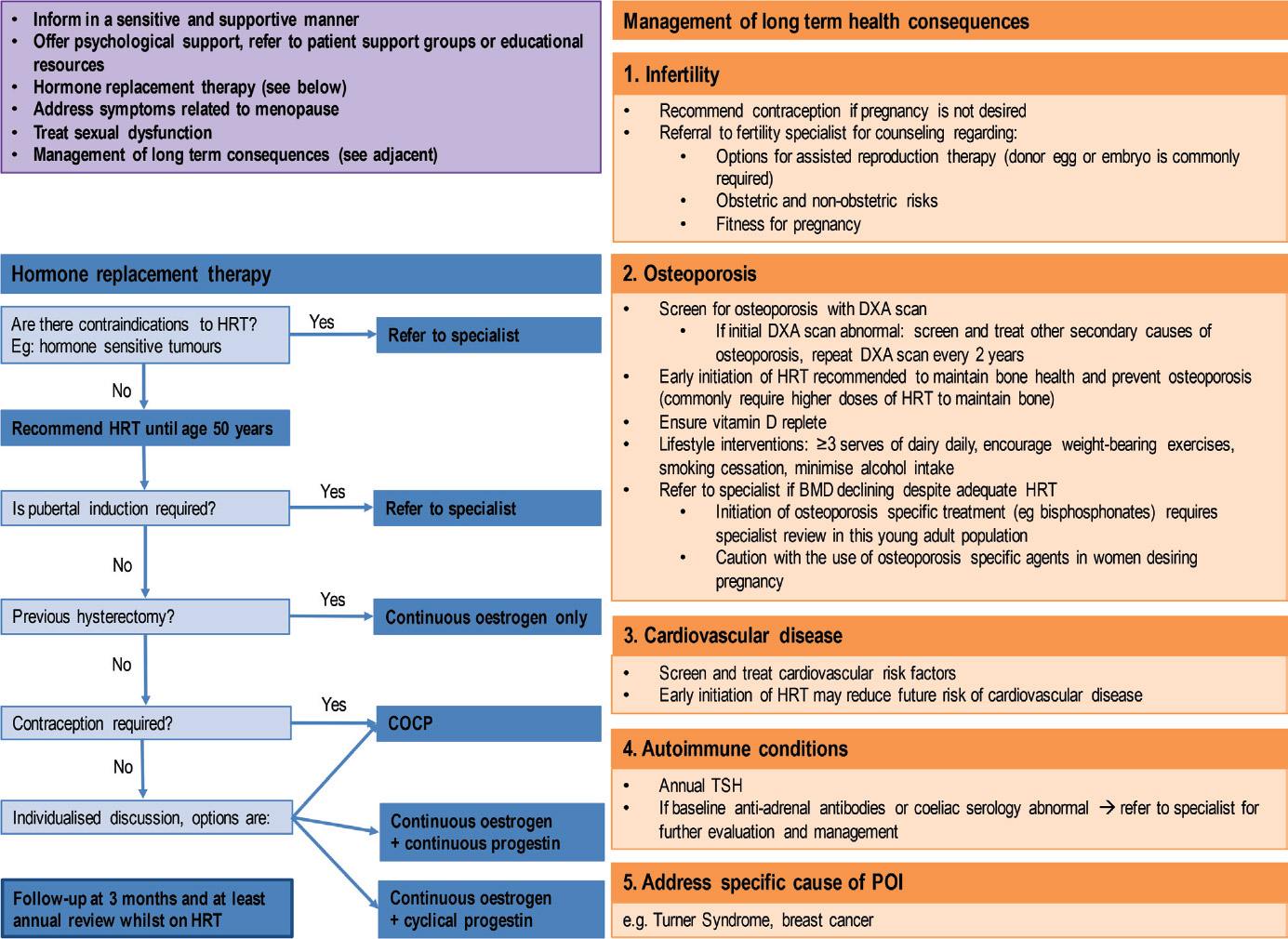
Figure 3. Premature ovarian insufficiency management guidelines
Abbreviations: POI = premature ovarian insufficiency; HRT = hormone replacement therapy; COCP = combined oral contraceptive pill;
DXA = dual energy X-ray absorptiometry; TSH = thyroid stimulating hormone
Case continued
Subsequent karyotype and Fragile X premutation testing are normal, as are the patient’s adrenocortical and thyroid antibodies, and coeliac serology. A pelvic ultrasound demonstrates a normal uterus with small ovaries and an absence of follicles. Dual energy X-ray absorptiometry (DEXA) shows low bone mineral density (BMD) at the lumbar spine with a Z-score of –2.1 (T-score –2.1), and a Z-score of –1.7 (T-score –1.7) at the hip. Calcium, phosphate, magnesium and 25-hydroxy-vitamin D levels are normal. She has no other known cardiovascular risk factors, and her fasting glucose level and lipid profile are normal.
Long-term consequences
Women with POI are at risk of complications relating to the underlying cause of POI, such as breast cancer recurrence or complications of Turner syndrome, but are also at risk of developing long-term consequences from POI itself. The key long-term consequences are shown in Figure 1 and summarised below. POI is an independent risk factor for CVD,16 with increased premature coronary artery disease17 and CVD mortality.18,19 Although the exact mechanism is unclear, adverse changes in lipid profile20 and impaired endothelial function21 related to oestrogen deficiency may lead to premature atherosclerosis. Observational studies suggest that there is an increased risk of diabetes following surgical menopause.19
Osteoporosis is considered one of the most feared consequences by patients with POI.13 The prevalence of osteoporosis is estimated to be 8–14% in women with POI,22,23 and bone loss of up to 26% at the lumbar spine, compared with a control population, has been reported.24 The risk of osteoporosis is associated with the presence, degree and duration of oestrogen deficiency, and although the prevalence of fractures is unknown, it is expected to be high.1
Studies have also suggested a possible link between cognitive impairment, dementia and Parkinson’s disease and POI.25 In Turner syndrome, any associated intellectual and cognitive impairment may reflect both the underlying genetic abnormality and ovarian insufficiency.
Women with POI are at risk of premature mortality,18 largely due to CVD, and may be worsened by the presence of other modifiable CVD risk factors. It is important to reassure women that POI is associated with a reduced risk of breast cancer.26
Management
The diagnosis of POI can be extremely distressing for women. Several consultations may be required to provide emotional and psychological support regarding the diagnosis, and to address the multisystem approach needed for optimal care.
Case continued
After a long discussion with the patient about the diagnosis of POI, its clinical implications and her plans for pregnancy, the importance of oestrogen therapy until the average age of menopause (approximately 51 years of age) is explained. Our patient commences transdermal combined continuous HRT (containing 50 µg oestradiol and 140 µg norethisterone). The consequences of POI on future fertility are particularly distressing for her, and she is counselled on the possibility of spontaneous ovulation in POI. Importantly, she is advised that her current HRT is not contraceptive and, therefore, contraception is required should she not wish to become pregnant. The patient is also referred to a psychologist for counselling.
Following almost two years of HRT, a repeat DEXA demonstrates a 12% increase in BMD at the lumbar spine and a 9% increase at the hip. Our patient continues to see her psychologist, and is managed by a multidisciplinary outpatient team and her GP.
Management of women with POI requires a multidisciplinary team approach to address the psychological impact of the diagnosis, initiate HRT (if not contraindicated) and/or contraceptive options, manage menopausal symptoms and long-term consequences, address the underlying cause of POI, and infertility treatment. Comprehensive guidelines on the management of women with POI were recently published,1 and key points from this review are highlighted below.
Psychological support
Referral for psychological support should be considered, and women should be made aware of available support groups and educational resources. A list of educational resources is provided at the end of the article.
Lifestyle modification
Menopausal symptoms and reduction of CVD or osteoporosis risk can be addressed by dietary and lifestyle modification.1,27 These include smoking cessation, healthy weight maintenance, adhering to recommended alcohol intake, regular weight-bearing exercises, adequate calcium intake and sufficient vitamin D levels. Refer to AMS and Jean Hailes websites listed below.
Hormone replacement therapy
The IMS and ESHRE guidelines recommend that HRT be initiated early in all women diagnosed with POI (unless contraindicated) and continued until the natural age of menopause (approximately 51 years of age).1,15 Findings from the Women’s Health Initiative study28 do not apply to young women with POI who have a reduced risk of breast cancer but an increased risk of CVD, osteoporosis and premature mortality.1,10
Currently, there is no conclusive evidence regarding the optimal HRT regimen.1 Various factors require consideration in the selection of HRT preparations, and HRT should be individualised to improve adherence, taking
into account women’s wishes. The usual considerations regarding the choice of HRT or COCP applies to women with POI. For example, oestrogen-only therapy in women is recommended for women who have had a hysterectomy, cyclical progestin combined with oestrogen for women who prefer monthly withdrawal bleeds (or continuous combined therapy in those who do not) and transdermal oestrogen for women at increased risk of venous thromboembolism, migraines or liver disease. HRT may be preferable to COCP |for optimisation of bone health, but must be weighed against the need for contraception.
Women with primary amenorrhoea requiring pubertal induction, contraindications to HRT (hormone-sensitive tumours), or a history of thrombophilia or endometriosis require specialist referral. Figure 3 includes an algorithm to assist with this decision-making process, and a list of available HRT preparations can be found online (refer to AMS’s Guide to equivalent HRT/MHT dose). Initial doses may be low and up-titrated to achieve symptom control, although higher oestrogen doses may be required in POI for symptomatic management and maintenance of bone health.1 Complementary therapies are not recommended as data regarding efficacy and safety are lacking in the POI population.1
As HRT is not contraceptive, counselling regarding contraceptive options is important for women not desiring pregnancy. COCP can provide both hormone replacement and contraception and, if prescribed, women should be advised to take it continuously or long cycle, without the inactive pills, to avoid intermittent periods of symptomatic oestrogen deprivation.1 Other forms of contraception (eg Implanon, progesterone-only pills, depot medroxyprogesterone, intrauterine devices [IUDs]) provide contraception but not oestrogen replacement. An option would be to use transdermal oestrogen with the levonorgestrel IUD, thereby providing contraception, symptom management and prevention of long-term sequelae.
Women should be reviewed frequently while the dose of HRT is titrated. Once a maintenance dose is established, consultations should occur at least annually to monitor for symptom control and to complete an annual complication screen.
Sexual dysfunction
A sexual health history is important, as women are often reluctant to disclose sexual dysfunction. Key treatment issues to consider include optimisation of HRT, treatment of urogenital symptoms (eg vaginal oestrogen or lubricant to treat dyspareunia), review of medications that can have an impact on sexual function (eg antidepressants, aromatase inhibitors), counselling, and referral to sexual health clinics.3 Low female androgen levels (measured using sensitive testosterone assay) in POI may contribute to sexual dysfunction. Some women may benefit from consideration of testosterone therapy in this setting, but there is insufficient evidence regarding the efficacy and long-term safety in women with POI to support the routine use of testosterone.1,3
Long-term consequences
Principles for managing the potential long-term sequelae of POI are listed in Figure 3, and include the issues of infertility, CVD and osteoporosis risk assessment; consideration of specialist referral; autoimmune screening; and management of the underlying cause of POI (eg Turner syndrome, autoimmune conditions).
Education
Resources for health professionals
Resources for patients
Conclusion
The diagnosis of POI is often challenging for health practitioners, and traumatic for affected women. In the primary care setting, evaluation should include confirmation of the diagnosis of POI, the underlying cause, and associated symptoms and consequences. First-line therapy includes oestrogen replacement (eg HRT, COCP), and multidisciplinary team involvement is often required. Women require long-term follow-up by GPs to monitor therapies, and also to address complication screening.
Authors
Hanh H Nguyen MBBS, BMedSci, FRACP, Endocrinology Research Fellow, Department of Medicine, School of Clinical Sciences, Monash University, Clayton, Vic; and Endocrinologist, Department of Endocrinology, Monash Health, Clayton, Vic. hanh.nguyen@monash.edu
Frances Milat MBBS (Hons), MD, FRACP, Endocrinologist, Head of Metabolic Bone Services, Department of Endocrinology, Monash Health, Clayton, Vic; Head, Metabolic Bone Research Group, Hudson Institute of Medical Research, Clayton, Vic; and Adjunct Clinical Associate Professor, School of Clinical Sciences, Monash University, Clayton, Vic
Amanda Vincent MBBS, BMedSci (Hons), PhD, FRACP, Endocrinologist, Department of Endocrinology, Monash Health, Clayton, Vic; Research Fellow, Monash Centre for Health Research and Implementation, School of Public Health and Preventive Medicine, Monash University, Clayton, Vic; and Adjunct Clinical Associate Professor, School of Public Health and Preventive Medicine, Monash University, Clayton Vic
Funding and competing interests: Amanda Vincent has received personal fees from Novo Nordisk, and research grants and personal fees from Amgen, outside the submitted work. Hanh Nguyen and Frances Milat have no disclosures.
Provenance and peer review: Commissioned, externally peer reviewed.