It is estimated that 30–70% of the 1.1 billion overseas travellers in 2014 experienced traveller’s diarrhoea (TD). This condition is defined as the passage of loose stools three or more times in less than 24 hours during or shortly after returning from overseas travel.1 Diarrhoea is classified as acute (fewer than two weeks), persistent (two to four weeks) or chronic (four weeks or longer). Acute diarrhoea accounts for the majority of TD cases, whereas persistent and chronic diarrhoea are less common, with estimated prevalences of 3% and 1–2% respectively.1–3 Most cases of TD are caused by bacteria or viruses and resolve within days, but some individuals experience persistent gastrointestinal (GI) symptoms.2 Causes of ongoing GI symptoms in returned travellers may be categorised as:3
- parasitic infections, mainly Giardia and Entamoeba histolytica
- unmasked GI disease such as inflammatory bowel disease (IBD) or malignancy
- post-infectious sequelae, including post-infectious irritable bowel syndrome (PI-IBS).
TD is an increasingly recognised cause of serious short-term and long-term GI sequelae.4 Proposed mechanisms include dysregulation of local immune and neuroendocrine systems, and alterations in the gut microbiome. Changes in the gut microbiome may be induced by the initial episode of acute diarrhoea5 and/or treatment with antimicrobial therapy.6
Chronic GI symptoms represent a significant clinical and public health issue, and are a common presentation in the primary care setting.7 Among returned travellers, this often follows an acute travel-related episode of diarrhoea, with considerable associated costs related to disability, reduced quality of life, lost income and healthcare expenditures.8 However, the burden of chronic illnesses associated with TD is often under-recognised.
Despite reviews and expert opinions on the management of chronic diarrhoea in returned travellers,3 there is a lack of clear evidence on optimal management to support the guidelines. Most studies have either focused on acute TD rather than long-term symptoms,9–11 or restricted their analysis to the incidence and aetiologies of ongoing GI symptoms rather than management.12,13 Therefore, research is needed to generate evidence and inform management protocols for these patients.
The aim of this study was to characterise the aetiologies, investigations and treatments of patients with persistent GI symptoms following travel who were referred to a specialist infectious diseases service.
Methods
We conducted a retrospective review of the medical files of patients who attended the Victorian Infectious Disease Service (VIDS), an Australian tertiary-level infectious diseases clinic at the Royal Melbourne Hospital, from January 2013 to March 2015. Patients were included in the study if they had:
- travelled overseas in the previous two years
- ongoing GI symptoms lasting longer than two weeks, and
- acquired their illness outside Australia.
An initial episode of acute TD was defined as the onset of diarrhoea during or within 10 days of returning to Australia from travelling, and lasting fewer than two weeks.1 Travel zone risk level was based on TD prevalence according to travel destination by the Centers for Disease Control and Prevention (CDC; Table 1). Patients were recorded as having abnormal investigation findings if tests performed prior to or after referral to VIDS consultation were abnormal. Diagnoses were assigned by VIDS physicians on the basis of microbiology, clinical features, investigation results or response to treatment. Outcomes were recorded as resolution of symptoms and clinical state at discharge. If patients had not been discharged by the end of the study period, their outcome was recorded as unknown.
Data were collected retrospectively by a review of electronic and hard copy medical record files to extract information on patients’:
- demographics (age, sex, country of birth and current residence)
- travel histories (reason for travel, destination and duration)
- reason for seeking medical care
- clinical symptoms and examination findings
- results of investigations
- diagnoses, treatment and outcomes.
Descriptive analysis was performed and comparisons made between diagnostic groups (Excel, Microsoft Corporation, 2013). This study was conducted as an internal quality assurance audit and hence human research ethics committee approval was not required.
Table 1. Comparison of demographic and travel characteristics, initial TD and presentation to VIDS between diagnostic groups
|
|
Diagnosis
|
|---|
| |
Bacterial/viral infection |
Parasitic infection |
PI-IBS |
Other GI pathology* |
Other† |
Total |
| n (%) |
5 (8) |
31 (47) |
12 (18) |
10 (16) |
7 (11) |
65 (100) |
|
Demographics
|
|---|
| Male sex |
2 |
18 |
5 |
7 |
3 |
35 (100) |
| Age in years – median (IQR) |
40 (29–40) |
36 (29–41) |
38 (28–46) |
34 (31–34) |
36 (29–38) |
35 (29–42) |
|
Traveller type‡
|
|---|
| Visiting family and relatives |
0 |
8 |
0 |
3 |
0 |
11 |
| Tourist |
4 |
11 |
11 |
3 |
6 |
35 |
| Long-term§ |
1 |
12 |
1 |
3 |
1 |
18 |
|
Travel zone risk||#
|
|---|
| High |
4 |
23 |
9 |
6 |
6 |
48 |
| Medium |
0 |
3 |
2 |
2 |
1 |
8 |
| Low |
0 |
3 |
1 |
1 |
0 |
5 |
|
Travel duration**
|
|---|
| Number of days – median (IQR) |
35 (30–56) |
60 (36–183) |
75 (22–183) |
30 (23–92) |
67 (26–197) |
54 (28–176) |
| Travelled >30 days |
2 |
12 |
7 |
2 |
3 |
26 |
|
Pre-VIDS‡
|
|---|
| Initial TD (number resolved) |
4 (3) |
10 (6) |
6 (4) |
5 (3) |
5 (5) |
30 (21) |
| Pre-VIDS treatment |
3 |
16 |
6 |
5 |
4 |
34 |
| Number of treatments – median (IQR) |
1 (0–1) |
0.5 (0–1) |
0.5 (0–2) |
0.5 (0–1) |
1 (0.5–1) |
1 (0–1) |
|
Time to presentation to VIDS††
|
|---|
| Number of days – Median (IQR) |
51 (29–89) |
56 (24–445) |
195 (67–544) |
71 (45–132) |
98 (67–248) |
88 (29–265) |
| Presented within four weeks of return |
2 |
8 |
1 |
2 |
1 |
14 |
|
IQR, inter-quartile range; PI−IBS, post-infection irritable bowel syndrome; GI, gastrointestinal; VIDS, Victorian Infectious Diseases Service
*Includes IBD, eosinophilic colitis, rectal carcinoma and non-infectious liver pathology
†Includes rheumatological pathology and unknown cause for symptoms at discharge
§Includes business, volunteer, missionary and migrating travellers
||Defined based on prevalence rates of TD as a function of travel destination by the Centers for Disease Control and Prevention. Low-risk areas include Northern and Western Europe, North America, New Zealand and Japan. Medium-risk areas include Southern and Eastern Europe, Russia, the Middle East, South Africa and the Caribbean. High-risk areas include Asia, Sub-Saharan Africa, India and Latin America
Number of unknown: ‡1; #4; **23; ††13
|
Results
Data were recorded for 65 patients (Table 1), accounting for 159 visits (1.5% of all VIDS travel clinic outpatient consultations during the study period). Fifty (77%) patients were under 40 years of age. Most were tourists travelling for longer than 30 days to high-risk zones (Table 1) in the Asia-Pacific region (34 patients) or Africa (13 patients). Of the 65 patients, 36 (55%) were diagnosed with an infectious cause, mostly parasitic (Table 1). Non-infectious diagnoses included PI-IBS (12 cases, 18%), rheumatological diseases (five cases), IBD (two cases) and rectal adenocarcinoma (one case). Thirty (46%) patients without parasitic infection reported an initial episode of acute TD, which mostly abated (n = 21, 70%), yet lingering GI symptoms prompted referral. Overall, of 52 patients with known time between return and presentation, 38 (73%) presented more than four weeks following their return to Australia.
Diarrhoea and abdominal pain were the most frequently reported symptoms. Most patients with a fever were diagnosed with an infectious aetiology (Figure 1). The duration of diarrhoea and abdominal pain was longest in the parasitic infection and PI-IBS groups (maximum duration of longer than 2.5 years). Significant impact on daily activities was reported in 14 cases, consisting of hospitalisations and time off work.
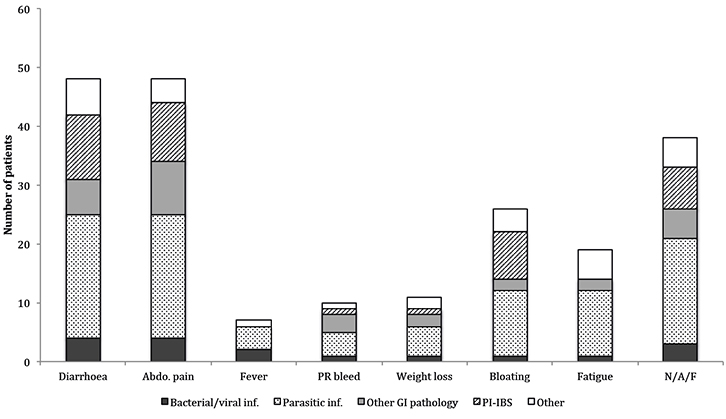 |
Figure 1. Prevalence of diagnostic groups by presenting symptoms
abdo., abdominal; inf., infection; PR, per rectum; N/A/F, patient presents with one of more of nausea/ vomiting, anorexia and food intolerance |
Across all diagnostic groups, 62 (95%) patients had microscopy testing for faecal oocyte, cyst and parasite (O/C/P), of which 37 (60%) were positive. In patients with parasitic infections, 24/30 (80%) O/C/P tests were positive (Figure 2) and the remaining 20% were diagnosed using polymerase chain reaction (PCR), antigen and/or serological tests. In one case of discordant results (O/C/P positive for Entamoeba spp. and PCR negative), treatment was administered and resulted in symptom resolution.
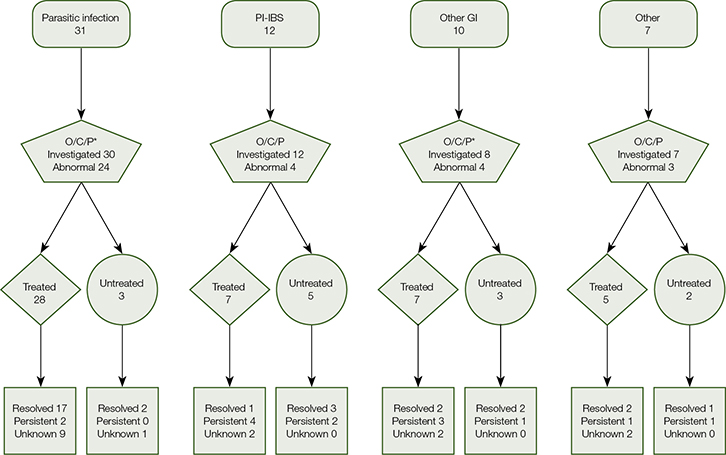 |
Figure 2. Flowchart of patient treatment and outcome at discharge by diagnostic groups (bacterial/viral infection excluded: H. pylori [two cases] C. difficile [two cases], Norovirus [one case])
O/C/P, oocytes/cysts/parasites; PI–IBS, post-infection irritable bowel syndrome; GI, gastrointestinal
*Respectively, one and two patients in these groups did not have documented O/C/P testing |
Seventeen patients whose first faecal sample was negative had a second sample taken. Of these patients, nine (52%) had a positive microbiological result on repeat testing. This suggests there was an incremental benefit in ordering more than one one faecal test before commencing treatment. Faecal PCR testing was performed when species identification could not be confirmed on microscopy alone, such as for Entamoeba histolytica (n = 6). Other investigations included coeliac disease serology, thyroid function tests, malabsorption markers and human immunodeficiency virus (HIV) testing (all negative). Imaging studies and upper/lower GI endoscopy were ordered if indicated, and yielded positive findings in 11/23 cases (48%). Referral to gastroenterology services was more frequent among patients without infectious causes identified (n =16/25, 47%).
Prior to VIDS referral, 34 of the 65
patients (52%) received antimicrobial treatment (most commonly metronidazole 400 mg, azithromycin 500 mg or doxycycline 200 mg). Fifty-two patients (80%) received therapy at VIDS (Figure 2). All patients with microbiological evidence of infection received targeted treatment, except those with Blastocystis hominis, of whom only nine out of 13 were treated. Of 26 patients with negative faecal samples, ten (38%) received empirical antimicrobial treatment (Table 2). Among 35 patients with known response, 24 (69%) obtained significant symptomatic improvement or resolution following their last treatment (Figure 2), including six of eight patients with B. hominis and known response, two of six patients with Dientamoeba fragilis infection and known response, and one of seven empirically treated with known response (Table 2). Among 24 patients who responded to antimicrobial treatment, 15 had post-treatment faecal testing and 13 had documented microbiological cure.
Table 2. Microbiological evidence of infection, antimicrobial therapy and response to therapy for patients with confirmed or suspected parasitic infection
|
| Faecal pathogens* | Positive | Overall** | Met | Tin | Tin/Par | Par | Others |
|---|
| B. hominis |
13 |
9 (6)† |
2 (0) |
6 (4)† |
1 (1) |
1 (1) |
– |
| B. hominis and D. fragilis |
4 |
4 (2) |
3 (1) |
– |
– |
1 (1) |
1 (0)‡ |
| D. fragilis |
3 |
6 (2) |
2 (0) |
2 (0) |
4 (1) |
1 (1) |
– |
| Giardia |
4 |
4 (2)† |
– |
4 (2)† |
– |
– |
1 (0)§ |
| E. histolytica |
6 |
3 (2)† |
2 (2) |
– |
1 (0)† |
2 (2) |
– |
| Taenia sp. |
5 |
5 (4) |
– |
– |
– |
– |
5 (4)†||; 2 (0)‡ |
| T. trichuria |
1 |
1 (1) |
– |
– |
– |
– |
1 (1)# |
| C. sinensis/Opisthorchis |
1 |
1 (1) |
– |
– |
– |
– |
1 (1)|| |
| Suspected parasitic infection (negative microbiological findings) |
0 |
10 (1)†† |
3 (1) |
8 (0)†† |
– |
1 (–)† |
2 (0)‡ |
|
Results shown are number treated (number resolved)
*Bacterial/viral infection excluded: H. pylori (two cases) C. difficile (two cases), Norovirus (one case); **Had ≥1 antimicrobial
Number of unknown: †1; ††3
Other antimicrobial agents: ‡Albendazole; §Nitazoxanide; ||Praziquantel; #Mebendazole
Met, metronidazole; Tin, tinidazole; Par, paromycin
|
Where outcome at discharge was known, resolved symptoms were reported by 90% (n =19/21) of those with parasitic infections, in contrast to 46% (n = 11/24) of other diagnostic groups (Figure 2). Of 13 patients who did not receive antimicrobial therapy, nine were discharged symptom-free following supportive treatment (two patients), dietary modification (four patients) or illness resolving spontaneously (three patients). Of the 15 patients discharged with persistent symptoms (including one patient in the bacterial/viral diagnostic group not shown in Figure 2), nine were referred to another clinic (mainly gastroenterology).
Ten patients were prescribed metronidazole (400 mg three times daily for seven days) prior to VIDS referral, without symptomatic improvement (Table 2). Following referral, metronidazole was prescribed only to treat Clostridium difficile and E. histolytica infections (the latter followed by paromomycin to eradicate cysts in the GI tract), and as a second-line treatment for Helicobacter pylori infections. Tinidazole (2 g as single dose, first-line treatment of giardiasis)14 was the most commonly prescribed antibiotic at VIDS; however, it was rarely prescribed prior to referral. Twenty-one patients were treated with tinidazole as a single agent, of whom 50% (8 out of 16 patients) of those with a known response improved (Table 2).
Paromomycin as a sole agent was prescribed prior to VIDS in only one case, where it was prescribed to a patient while they were overseas. Seven patients were treated with paromomycin (500 mg, three times daily for one week)14 as a single agent, of whom 83% (5 out of 6 patients) with a known response improved. Six patients received a combination of tinidazole and paramomycin, and 40% (2 out of 5 patients) with a known outcome improved. Praziquantel
(10 mg/kg as a single dose)14 was prescribed for five cases of Taenia infection, with good symptom resolution (Table 2).
Discussion
Persistent GI symptoms post-travel are not uncommon15 and have myriad causes. In our patient group, the most common diagnosis was parasitic infection (mostly protozoan). C. difficile was also an important bacterial cause for symptoms lasting longer than two weeks. Its prevalence is rising among returned travellers, thus detection through toxin assay should be requested in this population.12,16 Among non-infectious causes, several patients reported a preceding episode of TD, indicating TD may act as a trigger for prolonged GI symptoms.4,5 This highlights PI-IBS as an increasingly recognised cause of persistent symptoms in returned travellers with previous TD, with a reported prevalence of 4–17%.13,17,18
Initial management of prolonged post-travel GI symptoms by general practitioners (GPs) often involves faecal testing followed by directed or empirical treatment. A subset of patients with persistent GI symptoms was referred to specialist services. A history of overseas travel is more likely to prompt referral to an infectious diseases specialist to exclude infection.19 While the management of chronic GI symptoms post-travel has been discussed in the literature,15,20,21 clear guidelines based on empirical evidence specifically addressing investigations, treatment and when to refer are lacking. Auditing the causes, management and outcomes of returned travellers with ongoing GI symptoms referred to a tertiary infectious diseases clinic is a valuable step towards developing such guidelines.
Our study highlights several key points relevant to GPs caring for returned travellers with prolonged GI symptoms. First, abdominal pain was as frequently reported as diarrhoea13 and may warrant investigations and treatment of an infectious cause even when diarrhoea is not present. Second, the investigation of choice remains faecal O/C/P, with an incremental benefit in conducting more than one faecal test before commencing treatment. Parasites can be intermittently shed; hence, a single negative result does not exclude their presence. Analysis of a second specimen is beneficial when symptoms persist and a cause has not been identified.22 However, Medicare Benefits Schedule (MBS) rebate for stool examination of O/C/P is provided only once in any seven-day period.23
The superiority of PCR over microscopy remains a matter of debate, as the latter enables detection of parasites not included in PCR panels.24 Nonetheless, given its higher sensitivity and faster turnaround time, PCR is already available in a number of pathology laboratories across Australia. It is likely to become the mainstay for the diagnosis and follow-up of enteric infections once reimbursement and logistic systems become established.24 However, clinically non-significant positive results sometimes arise, so PCR must be interpreted in the context of clinical findings.
Third, management can be problematic for organisms where pathogenic potential is controversial and for which different subtypes may have varying degrees of pathogenicity25 (eg B. hominis and, to a lesser extent, D. fragilis and Giardia26,27). Also, management can be challenging when no pathogen is detected. Consistent with other studies,28,29 we observed some discrepancies between microbiological cure and clinical symptoms. However, the positive response of some patients harbouring these organisms suggests that a trial of treatment is warranted in the setting of congruent symptoms.25 Insofar as most enteric infections are acquired by ingestion of contaminated food or water, co-infection with multiple pathogens is possible. Thus, microbial treatment may be acting on unidentified pathogens.3
Fourth, metronidazole is often recommended as first-line therapy for the empirical treatment of suspected parasitic infections.19,21 Our observation of patients referred following failed metronidazole treatment could be due to selection bias rather than lack of efficacy. Although metronidazole and tinidazole have equivalent efficacy for many indications, tinidazole is required for a shorter duration and is generally better tolerated.30 Our results suggest that GPs could consider tinidazole as first-line treatment in this patient group, or as second-line therapy for those who have not responded to metronidazole.
A trial of multiple antimicrobial agents may, however, be necessary, as observed in this study. For patients who do not respond to tinidazole, an alternative agent, such as paromomycin, should be considered. Paromomycin can be effective as a single agent for a number of parasitic infections,31 but it is not in widespread use. This is partly because its access is restricted to authorised prescribers on a case-by-case basis.32 Patients may therefore need to be referred to an infectious diseases service for such therapy because GPs cannot prescribe paromomycin.
Our results suggest that referral to an infectious diseases service is recommended in cases of diagnostic uncertainty (eg negative faecal tests results or detection of a controversial organism), failed treatment with metronidazole, and for specialist input on the management of severe and/or unusual infections.
This study reports on a highly selected patient group, which limits generalisability. Nevertheless, it is instructive regarding the management of prolonged post-travel GI symptoms. Further research is necessary to:
- determine the optimal number of diagnostic faecal tests
- evaluate the role of routine PCR
- better ascertain the relationship between treatment and cure (both symptomatic and microbiological) for organisms with variable pathogenicity
- compare treatment with agents such as tinidazole and paromomycin.
Implications for general practice
- Abdominal pain is as frequent as diarrhoea in returned travellers, and may warrant investigations and treatment of an infectious cause without the presence of diarrhoea.
- There are incremental benefits in ordering at least two faecal samples in symptomatic patients with a first negative faecal specimen and persistent symptoms.
- Referral to an infectious diseases service in cases of diagnostic uncertainty and/or failed management should be considered.
- If metronidazole treatment has failed, a trial of tinidazole before referral to an infectious diseases specialist may be beneficial.
Conclusion
Persistent GI symptoms post-travel are difficult to manage and a source of frustration for patients. Our results highlight the utility of ordering more than one faecal specimen for O/C/P examination, potential benefits of tinidazole use by GPs, and the role of specialist services for patients with uncertain diagnoses or complex and/or unusual organisms.
Authors
Noha Ferrah BSc, BBiomed, MD, Research Student, Victorian Infectious Disease Service, Royal Melbourne Hospital, Parkville, VIC. nohaferrah@gmail.com
Karin Leder MBBS, FRACP, PhD, MPH, DTMH, Professor, Head of Travel Medicine and Immigrant Health, Victorian Infectious Disease Service, Royal Melbourne Hospital, Parkville, VIC; Department of Epidemiology and Preventive Medicine, Monash University, The Alfred Centre, Melbourne, VIC
Katherine Gibney, MBBS, FRACP, MPH, Infectious Diseases Physician, Victorian Infectious Disease Service, Royal Melbourne Hospital, Parkville, VIC; Department of Epidemiology and Preventive Medicine, Monash University, The Alfred Centre, Melbourne, VIC
Competing interests: Karin Leder has received research grants from GSK and Sanofi, personal fees from Immuron, and other support from Sanofi and GSK, all outside the scope of the submitted work. Katherine Gibney notes that CSL contributed to the National Health and Medical Research Council (NHMRC)-Gustav Nossal postgraduate award.
Provenance and peer review: Not commissioned, externally peer reviewed.
Acknowledgments
The authors thank Dr Thomas Schulz (VIDS ID physician) for his assistance with creating the new ‘Gastrointestinal symptoms’ clinical audit research electronic health record (CAReHR) clinical problem page, Ms Elizabeth Matchett and Ms Paulette Manton (VIDS registered nurses) for their assistance with identifying and obtaining medical records of eligible patients, and Prof Beverley-Ann Biggs for reviewing the manuscript.