The Global Burden of Diseases study highlights musculoskeletal conditions as an emerging global issue that must be addressed in the coming decade.1 The authors found that in Australasia, musculoskeletal conditions account for 15.3% of the total burden of death and disability, just behind cancer (16.2%), and ahead of heart disease (13.8%), mental health and substance abuse.1
In Australia, osteoarthritis (OA) is self-reported by more than 1.4 million people (7.3% of the population)2 and is the tenth most commonly managed problem in general practice.2 OA exerts a significant burden on society through reductions in quality of life, diminished employment capacity and increased healthcare costs.
Australian guidelines indicate there is excellent evidence to support general practitioners (GPs) prescribing paracetamol in regular, divided doses to a maximum of 4 g/day as first-line pharmacological therapy for treating persistent pain in people with OA of the hip or knee, in addition to lifestyle measures such as exercise and weight management.2 It is recognised that paracetamol may not be as effective as non-steroidal anti-inflammatory drugs (NSAIDs); however, the lower risk of adverse events, particularly in the elderly and those on concomitant medications, makes paracetamol the most appropriate first-line analgesic for mild to moderate pain of OA.2
The term ‘pain ladder’ was coined by the World Health Organization (WHO) to describe its guideline for the use of drugs in the management of pain.3 The general principle is to start with first-step drugs, then climb the ladder if pain is still present. The WHO guidelines recommend prompt oral administration of drugs when pain occurs, starting with non-opioid drugs such as paracetamol, NSAIDs or cyclooxygenase-2 (COX-2) inhibitors. If complete pain relief is not achieved, then a mild opioid such as codeine phosphate or tramadol is added to the existing non-opioid regime. If this is insufficient, the mild opioid is replaced with a stronger opioid, such as morphine or oxycodone, while continuing the non-opioid therapy. The opioid dose escalates until the patient is pain free or at the maximum possible relief without intolerable side effects.
Since the initial WHO guidance, there have been significant changes in the understanding of pain. It is increasingly considered a physiological process that merits and deserves independent treatment. Raffa and others4 suggested that more modern best practice may be an analgesic ‘pyramid’. That is, the analgesic pyramid starts with non-opioid drugs such as paracetamol. Then, NSAIDs/COX-2 inhibitors can be used for a short period of time and at the lowest dose to manage flare up of OA. If pain relief is not achieved, then a mild opioid is added to the non-opioid regime. Strong opioids are added if, and only if, needed.
Prior to January 2016, only two products had Pharmaceutical Benefits Scheme (PBS)-restricted chronic OA listings: ‘chronic arthropathies’ for immediate-release paracetamol (IRP), and ‘relief of persistent pain associated with osteoarthritis’ for extended-release paracetamol (ERP). Both formulations have now been delisted from the PBS.
The longer duration of pain relief offered by an extended-release formulation may provide advantages over the immediate-release formulation in terms of compliance and patient preference.5 If there is a clinically important difference between the formulations, then we would expect the extended release formulation to be associated with lower analgesic use.
The aim of this study was to compare the usage patterns on the PBS of ERP with IRP in Australian patients with OA. The PBS listing of the IRP is 300 tablets (with four repeats) of paracetamol 500 mg (eg Panamax), whereas the ERP is 192 tablets (with five repeats) of paracetamol 665 mg (eg Panadol Osteo).
Methods
A retrospective cohort longitudinal analysis was performed on PBS pharmacy payment claims in a 10% random sample of the Australian population. The data were drawn from de-identified records held by the Department of Human Services. The OA paracetamol formulations listed on the PBS are both priced below the general patient co-payment and are not recorded for general patients. Hence, this study is restricted to patients described as long-term concessional (no general prescription during the previous five years).
Patients with concession cards who were prescribed OA paracetamol (ERP or IRP) between January 2009 and December 2010 were selected (age was restricted to 50–85 years). Patients were excluded if they were taking products on the PBS listed for rheumatoid arthritis, other autoimmune inflammatory conditions or cancer pain. All PBS scripts for analgesics for the selected patients in the time window were assessed. This did not include over-the-counter products.
The index event was the first script for ERP or IRP after 1 January 2009 and before 31 December 2010. A new initiation of paracetamol for OA was defined if there was no script for ERP or IRP in the previous 12 months of the index event.
To assess overall analgesic supply in that period, analgesic equivalent days (AED) was calculated using defined daily doses (DDD, the average maintenance dose per day for a drug used for its main indication in adults)4 for each patient as follows: AEDs = (strength x quantity x number of scripts)/DDD for each analgesic. Maximum daily dose (MDD) of 4.0 g/day of paracetamol (Therapeutic Goods Administration [TGA]-approved maximum daily dose) was used to calculate the AEDs of paracetamol. AEDs were summed over the first 12 months following the index script.
Comparisons were conducted for patient numbers, number of prescriptions, AED and number of analgesic classes between ERP and IRP. The interval between repeats for ERP and IRP was compared using a t-test for independent samples with a 5% two-sided alpha.
Persistence curves for patients newly initiated on ERP or IRP between October 2019 and September 2011 were generated using the time-to-event Cox proportional hazards model, adjusted for age group, sex and prescriber type. ‘Initiated to therapy’ was defined as an OA paracetamol script after no therapy in the previous 12 months, and ‘ceased therapy’ was defined as no OA paracetamol script for six consecutive months.
The PBS administrative database does not provide clinical information on reasons for discontinuation.
Results
Over the two-year period, there was a total of 74,114 concessional patients, which equates to 741,140 patients nationally, who were prescribed OA paracetamol. For all patients, around 65% of analgesic use was from OA paracetamol. A total of 46,255 of these concessional patients were newly initiated on ERP or IRP (ie they had not received ERP or IRP in the previous 12 months).
The majority of these 46,255 concessional patients were female (64%), aged 70–85 years (61%), and more than 95% were initiated on OA paracetamol by GPs. Users of IRP analgesics received 174,000 paracetamol scripts for 5.7 million AEDs; 23,000 scripts for NSAIDs and 54,000 scripts for COX-2 inhibitors for 2.1 million AEDs; and 57,000 scripts for opiod analgesics and 24,000 scripts for codeine combinations for 0.9 million AEDs.
Table 1. Comparison between ERP and IRP use by new patients
|
| | ERP users | IRP users | P value |
|---|
|
Demographics
|
| All patients collecting OA paracetamol (n) |
48,170 |
25,944 |
|
| Newly initiated OA patients (n) |
34,080 |
12,175 |
<0.001 |
| New initiations as a percentage of total patients (%) |
70.7% |
46.9% |
<0.001 |
| Mean age (years) |
71.0 |
72.7 |
<0.001 |
| Female (%) |
64.5% |
58.3% |
<0.001 |
| GP prescriber (%) |
94.5% |
96.8% |
<0.001 |
|
Analgesic ladder – mean AEDs for:
|
- Initial paracetamol formulation
|
115.7 |
109.1 |
<0.001 |
- Other paracetamol formulation
|
3.5 |
13.2 |
<0.001 |
- Total for all paracetamol
|
119.2 |
122.3 |
<0.005 |
|
|
33.2 |
26.8 |
<0.001 |
|
|
15.4 |
13.4 |
<0.001 |
|
|
5.5 |
5.5 |
ns |
|
|
10.5 |
14.2 |
<0.001 |
|
|
4.2 |
7.3 |
<0.001 |
| Total for 12 months |
187.9 |
189.5 |
ns |
| Proportion of total AEDs from paracetamol |
63.4% |
64.5% |
<0.05 |
| Mean number of classes of analgesics in 12 months |
1.85 |
1.77 |
<0.001 |
| Switch to other paracetamol formulation |
3.1% |
13.2% |
<0.001 |
|
Medication compliance
|
| Mean paracetamol prescriptions/patient in 12 months |
3.7 |
2.9 |
<0.001 |
| Mean interval between repeats (days) |
70.9 |
75.5 |
<0.001 |
| Did not collect the first repeat |
38.2% |
43.8% |
<0.001 |
| Median persistence (months) |
6 |
3 |
<0.05 |
| Hazard ratio after adjusting for confounding |
1.000 |
1.362 |
<0.001 |
| No gap in therapy after two years |
26.1% |
11.9% |
<0.001 |
|
AEDs, analgesic equivalent days; COX-2, cyclooxygenase-2; ERP, extended-release paracetamol; IRP, immediate-release paracetamol;
NSAIDs, non-steroidal anti-inflammatory drugs; OA, osteoarthritis
|
Total annual AED use was similar between ERP and IRP patients (188 and 189 respectively); however, ERP patients took 116 AEDs of their ERP formulation, compared with IRP patients who took 109 AEDs of their IRP formulation (Table 1). Consistent with the analgesic pyramid:
- around 65% of the AEDs were for paracetamol
- NSAIDs/COX-2 inhibitors accounted for 23% of AEDs
- opioid and codeine combination analgesics accounted for around 12% of AEDs.
On average, ERP patients took
NSAIDs/COX-2 inhibitors for eight more AEDs and opioid analgesics for six fewer AEDs than IRP patients in the first 12 months (Table 1).
OA patients averaged 1.8 classes of analgesics, which included 45% who collected paracetamol only, 25% who collected two types of analgesics and 30% who collected three or more types. The number of analgesic classes decreased with age (Table 1).
Around four times as many patients initiated on IRP (13.5%) switched to ERP in the first 12 months than those who switched from ERP to IRP (3.1%).
Of the newly initiated patients, 38.2% did not collect a repeat for ERP (95% confidence interval [CI]: 37.6–38.6%) within six months of the original script, compared with 43.8% for IRP (95% CI: 42.8–44.7%). Long-term continuous use at two years (where the patient took paracetamol regularly for two years with fewer than six months between refills) was 26.1% (95% CI: 25.4–26.8%) for ERP compared with 11.9% (95% CI: 10.8–13.1%) for IRP (Figure 1).
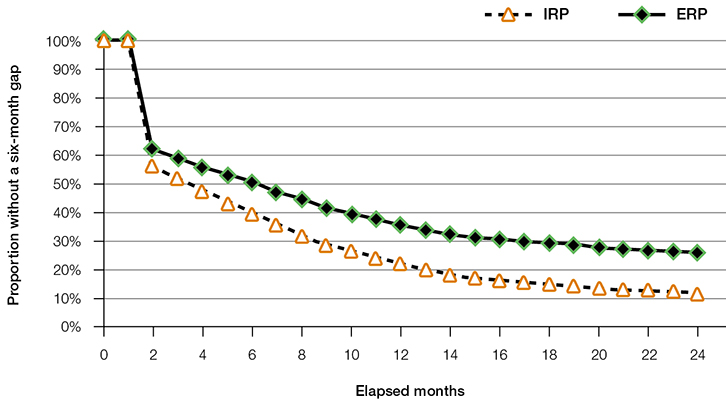 |
Figure 1. Persistence with ERP and IRP without a six-month gap
ERP, extended-release paracetamol; IRP, immediate-release paracetamol |
Discussion
The use of paracetamol by most patients with OA was episodic rather than continuous. Around 25% of ERP and 12% of IRP patients took the formulation continuously without a six-month break over the two years assessed. Analgesic use by patients with OA is consistent with the analgesic pyramid, with the majority of OA treatment taken being paracetamol (65% of AEDs), whereas opioid and codeine combination analgesics accounted for less than 12% of total AEDs. Almost half of the patients with OA seemed to manage their OA pain with paracetamol alone.
In a previous prospective study comparing IRP dosed four times daily with ERP dosed three times daily, the majority of patients preferred the ERP formulation.6 ERP provided better overall joint pain relief, and resulted in higher levels of satisfaction in Australian patients with OA of the knee.6
ERP concessional patients took a similar number of AEDs as IRP patients. When broken down by drug classes, ERP patients took fewer AEDs of opiod analgesics and more AEDs of NSAIDs/COX-2 inhibitors.
It is unclear exactly why ERP patients were less likely to move up the analgesic pyramid. A possible explanation is that the extended-release formulation gave better analgesic coverage with three times daily dosing than IRP. This lesser analgesic coverage may also explain the increased use of codeine combinations (three AEDs) and more potent opioid analgesics (three AEDs) with IRP.
It is also possible that the tablet burden and dose frequency with IRP may be an issue for some patients with OA. That is, taking two IRP tablets four times a day, compared with two ERP tablets three times a day, may explain the significant differences in patient compliance between two formulations, with 43.8% of ERP patients collecting at least one refill in the 12-month period, compared with 38.2% of IRP patients.
A recent meta-analysis of randomised controlled trials has questioned the efficacy of paracetamol in the management of patients with OA.7 However, if paracetamol were ineffective, then much greater use of the other analgesics would have been expected in this patient population than the 35% we observed. The current Australian Therapeutic Guidelines encourage patients to take regular paracetamol as an adjunct to non-pharmacological or other pharmacological strategies and accept that it rarely relieves pain completely but can modify its severity.8 By reducing pain in weight-bearing joints, patients may be more able to take regular exercise, which is an important factor in modifying disease prognosis. NSAIDs and opioids may be more effective in managing pain relief but are associated with a large burden of adverse effects, particularly in elderly people.
There are a number of limitations with this retrospective study:
- There is the potential for selection bias because more patients were initiated on ERP than on the IRP formulation in the two-year study period.
- There may be differences in the treatment populations because PBS restrictions were similar but not identical.
- It is assumed that if a script was dispensed, then it was taken by the patient.
- There are no details in the Medicare Benefits Schedule (MBS) data about the prescriptions collected by general PBS patients or about over-the-counter analgesics purchased at a pharmacy or grocery store.
Conclusion
Despite its limitations, this study, of a large sample of patients prescribed paracetamol to manage their OA pain, indicates that for the majority of patients, paracetamol forms the mainstay of their analgesic medication. Patients prescribed an ERP formulation may be more likely to use paracetamol regularly than patients prescribed an IRP formulation. This may in part explain the finding that ERP patients were less likely to move up the analgesic pyramid to be prescribed narcotic analgesics.
Implications for general practice
- Tablet burden with 4.0 g/day is lower for ERP with two tablets three times daily compared with two tablets four times daily with IRP.
- Patients prescribed an ERP formulation may be more likely to use OA paracetamol regularly than patients prescribed an IRP formulation.
- Patients may be less likely to move up the analgesic pyramid to use narcotic analgesics if they are prescribed ERP.
Authors
Michael Ortiz BPharm, PhD, Regional Director, SPMG Global and Conjoint Associate Professor, St Vincent’s Clinical School, University of New South Wales, Sydney, NSW. m.ortiz@unsw.edu.au
Gordon Calcino BA, GradDipMedStats, Director, HI Connections Pty Ltd, Woden, ACT
Fiona Dunagan MSc, PhD, Former Medical Affairs Manager, GlaxoSmithKline Consumer Healthcare, Sydney, NSW
Competing interests: The study was funded by GlaxoSmithKline, Sydney, New South Wales. Raw data for the study were supplied by the Department of Human Services. Michael Ortiz and Gordon Calcino have received payment for consultancy from GlaxoSmithKline as well as various other pharmaceutical companies. Michael Ortiz is the global regional director of SPMG (he was also formerly team leader pharmcaoepidemiology and pharmacoeconomics at NPS Medicinewise and is a former employee of Pfizer, Sanofi, Abbott and GlaxoSmithKline). Gordon Calcino is the director of HIS Connections Pty Ltd. Fiona Dunagan is an employee of GlaxoSmithKline and has shares in the company as part of her employment.
Provenance and peer review: Not commissioned, externally peer reviewed.