Twenty to forty per cent of pregnant women will experience bleeding during the first trimester of pregnancy.1 The major causes are miscarriage (10–20% of clinical pregnancies) and ectopic pregnancy
(1–2%).2 Bleeding in the very early weeks of pregnancy may be related to endometrial implantation. Rarer causes include cervical and vaginal lesions (eg malignancy, cervical ectropion, polyps, infection) and uterine infection. Gestational trophoblastic disease should always be considered, particularly in the setting of an abnormally raised serum human chorionic gonadotropin (hCG) or suggestive ultrasound findings. Establishing the site of the pregnancy is vital, as failure to correctly diagnose an ectopic can have potentially life-threatening consequences.
Assessment
The initial assessment of a woman with vaginal bleeding in early pregnancy must first consider haemodynamic stability and the degree of pain or bleeding. Immediate transfer to the emergency department is necessary in a haemodynamically unstable patient. It is important to recognise that young women may suffer significant blood loss before any signs of haemodynamic instability are evident.
The most likely diagnoses of a haemodynamically unstable patient with early pregnancy bleeding are a ruptured ectopic pregnancy an incomplete miscarriage with ‘cervical shock’ (parasympathetic stimulation caused by products in the cervical os leading to hypotension and bradycardia) or massive haemorrhage secondary to miscarriage.
A speculum examination must be performed and any products of conception (POC) removed from the cervical os. These patients may require urgent transfer to the operating theatre for a suction curettage, laparoscopy or laparotomy.
In the haemodynamically stable patient, a more detailed assessment can be undertaken in the community setting.
History
It is important to assess the likely gestational age of the pregnancy, the amount of blood loss and any associated pain symptoms. Syncope, chest pain and shortness of breath may point to anaemia from significant blood loss, and shoulder tip pain may be associated with intra-abdominal bleeding.
Risk factors for ectopic pregnancy include:1
- current use of an intrauterine device (IUD) or the minipill
- pregnancy as a result of assisted reproduction
- a past history of pelvic infection or sexually transmissible infections (STIs) or tubal surgery
- previous ectopic pregnancy.1
Cervical smear history is relevant, particularly if any abnormal bleeding has also been occurring outside of pregnancy. Certain medical conditions, such as poorly controlled diabetes and thyroid disease, are associated with an increased risk of miscarriage.3
Examination
Following initial assessment for any evidence of haemodynamic instability
and anaemia, abdominal examination
may reveal areas of tenderness, guarding or rigidity, and signs of distension. The
fundus will be palpable above the symphysis pubis when the uterus reaches the size appropriate for a 12-week gestation. It may be palpable earlier than this in the case of a multiple pregnancy, gestational trophoblastic disease (GTD), or if other pelvic or uterine masses such as fibroids or ovarian cysts are present.
Speculum examination is performed to assess the amount and origin of ongoing bleeding. The vagina and cervix should be inspected for other causes of bleeding (eg polyps). Tissue present in the open cervical os must always be removed and should be sent for histopathological examination to confirm retained POC. Bimanual examination allows assessment of uterine size, dilatation of the cervical os, pelvic tenderness and cervical motion tenderness.
Pelvic and cervical motion tenderness need immediate further investigation and discussion with a specialist.
Investigations
A combination of ultrasound assessment and measurement of serum hCG is required to determine the location and viability of an early pregnancy when the woman has presented with bleeding. Testing for maternal blood group and antibody status will determine the need for Rh D immunoglobulin administration.
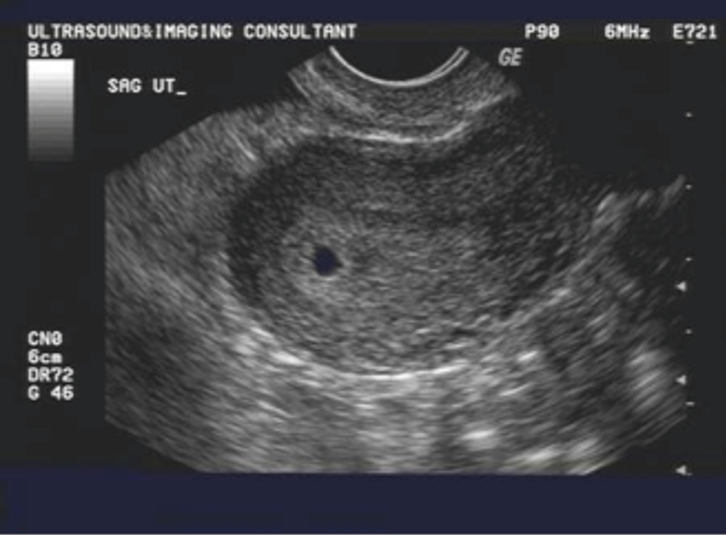 |
| Figure 1. Gestational sac within uterine cavity on TVS |
Serum hCG levels rise exponentially up to six to seven weeks of gestation, increasing by at least 66% every 48 hours.4 Following repeat measurements separated by 48–72 hours, a falling hCG is consistent with a non-viable pregnancy, but does not indicate whether the pregnancy is a failed intrauterine pregnancy (IUP), or an involuting ectopic. Plateaued or very slow to rise levels of hCG (<50% in 48 hours) are suggestive of an ectopic or non-viable intrauterine pregnancy.3 However, it should be noted that an apparently appropriately rising hCG is found in 21% of ectopic pregnancies.3
Ultrasonography for pregnancy assessment in the first trimester should be performed transvaginally by an experienced sonographer. On transvaginal ultrasound (TVS), a gestational sac will usually be visible from four weeks and three days after the last menstrual period,5 assuming the dates are correct and menstrual cycle is regular (Figure 1).
A non-viable pregnancy is diagnosed on ultrasound under either or both of the following circumstances:
- no live fetus visible in a gestational sac where the mean sac diameter (MSD) is >25 mm
- visible fetal pole with a crown rump length (CRL) of >7 mm, with no fetal heart activity after a period of observation of at least 30 seconds.5
If there is any doubt regarding the viability of the fetus, a second opinion or a review scan in one week is recommended. If a gestational sac is not visible in the uterus, the adnexa should be carefully examined for evidence of an ectopic pregnancy (Figure 2). An adnexal mass is the most common ultrasound finding in ectopic pregnancies, present in >88% of cases.6
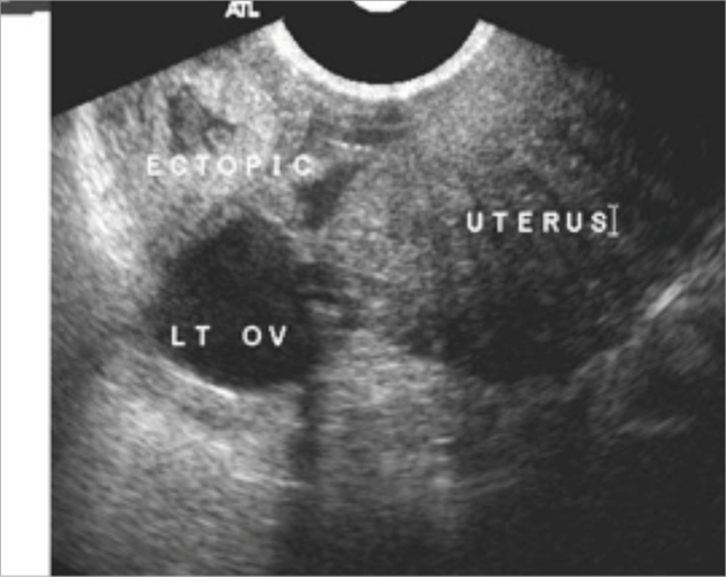 |
| Figure 2. Ectopic pregnancy on TVS |
The discriminatory zone is the serum hCG level above which a gestational sac should be visualised on TVS. In most institutions, this is set at 1500 or 2000 IU/L, although a number of variables, including skill of the sonographer and quality of the ultrasound, can alter the level. Below the hCG discriminatory zone, the diagnosis of a non-viable pregnancy can be made solely on the basis of an inappropriately rising hCG. Above the discriminatory zone, the diagnosis is based on the absence of evidence of an IUP on TVS. Table 1 gives examples of a discriminatory hCG of 2000 IU/L, with TVS findings and recommendations for management.
Table 1. Interpretation of hCG and TVS findings
|
| hCG/TVS in clinically stable women | Interpretation/recommendation |
|---|
| hCG <2000 IU/L |
Repeat TVS/hCG in 48–72 hours |
| hCG >2000 IU/L and TVS with no IUP, complex adnexal mass and/or free fluid |
High probability of ectopic pregnancy |
| hCG >2000 IU/L and TVS with no IUP and no abnormal findings |
Repeat TVS/ hCG in 48–72 hours |
| Declining or suboptimally rising hCG levels |
Indicates a non-viable pregnancy (ectopic or IUP), appropriate follow-up to ensure adequate resolution of either diagnosis |
| hCG, human chorionic gonadotropin; TVS, transvaginal ultrasound; IVP, intrauterine pregnancy |
Pregnancy of unknown location
If there are no signs on a TVS of an intra- or extra-uterine pregnancy, and no obvious retained POC are seen, the pregnancy is defined as a pregnancy of unknown location (PUL). Under these circumstances, the three possible scenarios are:5
- intrauterine pregnancy
- ectopic pregnancy
- failed PUL.
When interpreting the scan result of a woman with a PUL, there is evidence that serum hCG levels at zero and 48 hours are helpful in diagnosis of the ectopic location.5
Until the location is determined, a woman with a PUL could have an ectopic pregnancy. It is therefore important to re assess the woman if symptoms change. Women who have a plateauing hCG, or new or worsening clinical symptoms, require referral for specialist assessment. Clinical symptoms may necessitate admission to hospital while further investigations are undertaken.
Management of miscarriage
Table 2. Defintions of miscarriage
|
| Miscarriage | Pregnancy loss before 20 weeks’ gestation or fetal weight <400 g |
|---|
| Threatened |
Vaginal bleeding prior to 20 weeks’ gestation |
| Inevitable |
Passage of POC of a non-viable IUP occurring or expected to occur imminently |
| Incomplete |
Some retention of POC of a non-viable IUP |
| Missed |
Ultrasound scans diagnosis of a non-viable IUP in the absence of vaginal bleeding |
| Septic |
Miscarriage complicated by infection |
| Recurrent |
Three or more consecutive miscarriages |
| Complete |
Full expulsion of POC of an IUP |
| POC, products of conception; IVP, intrauterine pregnancy |
Several terms are used to describe clinical scenarios around the process of miscarriage. These are defined in Table 2.
Threatened miscarriage is treated expectantly. There is a 2.6 times increased risk of miscarriage later in the same pregnancy in cases of early threatened miscarriage, and 17% of women go on to have further complications in pregnancy (such as pre-term labour or intrauterine growth restriction).7 There is insufficient evidence to recommend progesterone administration in the setting of threatened miscarriage.8
For inevitable, incomplete and missed miscarriages, management options include expectant, medical and surgical treatments. Depending on the method chosen, follow-up will be required to ensure complete evacuation of the uterus. A complete miscarriage requires evaluation of any ongoing bleeding, with confirmation that the cervical os is closed, and, if necessary, a TVS to rule out retained POC.
Expectant management involves allowing the natural process of expulsion of uterine POC to occur without intervention. The woman must be informed regarding the expected length of the process, symptoms of pain and bleeding that she is likely to experience, and how to seek emergency medical assistance. For incomplete miscarriages, 60% of women experience complete expulsion of products in the ensuing two weeks and 90% by six to eight weeks.9 Missed miscarriages generally take longer to expel.
Ongoing review should occur at one to two weeks, and if pain and bleeding have ceased, a repeat serum hCG should be performed at three weeks. If this is positive, further assessment with serial hCG measurements, to ensure these fall to negative levels, or an ultrasound scan may be required to assess for retained POC.
If no bleeding or pain occurs within seven to 14 days of the initial consultation, repeat the ultrasound and further discuss all treatment options as appropriate.
Medical management involves the use of misoprostol (a prostaglandin E1 analogue), which has been shown to be highly effective for medical evacuation of the uterus given either vaginally or orally.10 Further research is required to determine the optimal dose and route of administration. Medical management should only be performed in a unit with experience in this form of management
Surgical evacuation is the treatment of choice for women with haemorrhage or sepsis. A woman may choose a surgical evacuation in order to avoid pain, bleeding and prolongation of the process. Medical management is contraindicated in some cases, such as for a woman on anticoagulant therapy. Complications of surgical evacuation include anaesthetic risks, haemorrhage, perforation, retained POC and endometritis.
The MIST trial,11 a large, randomised controlled trial, compared expectant, medical and surgical management options for the treatment of miscarriage. It showed comparable efficacy and no significant difference in infection rates (2–3%). The trial reported that unplanned hospital admissions were significantly increased in the expectant (49%) and medical (18%) groups, compared with the surgical group (8%). Surgical management was required in 44% of the expectant group and 13% of those given medication; 5% of the surgical group required a further surgical procedure.
Management of ectopic pregnancy
Ninety-five per cent of ectopic pregnancies are situated in the fallopian tube.12 Other, rarer sites include the cervix, ovary, other abdominal sites or in a caesarean section uterine scar. Rarely, an ectopic pregnancy may co-exist with an intrauterine pregnancy (heterotopic pregnancy).
Management options for tubal ectopic pregnancy include surgery (salpingectomy or salpingostomy), medical management with methotrexate, and possibly expectant management in a limited population of carefully selected cases, although no high-level evidence exists to recommend this approach.
Surgery is required for the haemodynamically unstable patient, those with evidence of rupture, after failed medical treatment and if contraindications to methotrexate therapy exist (including the possibility of non-compliance with follow-up). Some patients may choose surgery over medical management. Laparsocopic surgery should be performed whenever possible.13 A salpingectomy is usually performed unless the contralateral tube is damaged. A salpingostomy may result in a need for further treatment (4–15%)14 with methotrexate or a salpingectomy if follow-up hCG levels do not fall appropriately. In women who have had a salpingostomy, hCG levels should be measured weekly until negative due to the potential for retained pregnancy tissue in the affected tube. In the case of a salpingectomy, histological confirmation of a tubal pregnancy is usually all that is required.
About one-third of patients with ectopic pregnancy are suitable for medical management with methotrexate. These women should be haemodynamically stable, be able to comply with treatment and follow-up, ideally have an hCG <5000 IU/L (the greatest predictor of success)7,15 and an adnexal mass <3.5 cm with no fetal cardiac activity. Initial treatment is with a single intramuscular dose of methotrexate (50 mg/m2), with 14% of women requiring a further dose. Success rates are up to 85%, which is similar to salpingostomy.9 Up to 15% of women may require surgical intervention.
Ongoing fertility in the two to three years following surgical or medical treatment for ectopic pregnancy appears to be similar between methotrexate, salpingotomy and salpingectomy groups.15
Management of rhesus negative patients
Rh D immunoglobulin (RhIg) is indicated for the prevention of Rh D sensitisation in Rh D negative women. RhIg can be obtained through emergency departments or blood banks; 250 IU RhIg is required for a first trimester sensitising event such as miscarriage, ectopic pregnancy, termination of pregnancy and chorionic villous sampling. This should be given within 72 hours of the sensitising event, though administration of RhIg up to nine to 10 days later may provide some protection.1
Conclusion
Early pregnancy bleeding can cause great anxiety and distress for a woman, her partner and family, especially where a diagnosis of a non-viable pregnancy is made. It is important that the situation is dealt with both safely and sensitively and that the woman and her family are well supported throughout this time. Most tertiary and many regional hospitals now run early pregnancy assessment clinics that can assist GPs in the management of complications in the first trimester.
Author
Carol Breeze MBChB, FRANZCOG, Staff Specialist, Obstetrics and Gynaecology, Cairns Hospital, Lecturer, Obstetrics and Gynaecology, James Cook University, Townsville, QLD. Carol.Breeze@health.qld.gov.au
Competing interests: None.
Provenance and peer review: Commissioned, externally peer reviewed.