Sporotrichosis, traditionally known as ‘rose-handlers’ disease’, is a fungal infection caused by the dimorphic fungus Sporothrix schenckii.1 The infection is usually limited to the skin and typically presents as subacute or chronic skin lesions at the site of fungal inoculation (mostly on the arms or legs) following a breach in skin integrity.1 The infection may track along dermal lymphatics, forming superficial skin nodules (nodular lymphangitis) that have a characteristic sporotrichoid appearance.2 Disseminated disease or mortality occurs in rare cases.3 Person-to-person transmission and zoonotic spread have also been reported.4–6
The fungus has a ubiquitous, global distribution. It inhabits organic matter, including decaying vegetation, wood, hay, moss, fruits, plants and soil.3,7 Infection can either be sporadic or cause local outbreaks.3 The true incidence in Australia is unknown as the condition is not notifiable. Tropical climates with high temperatures and humidity favour the growth of this fungus.3 In Australia, geographical clusters have occurred in warm, moist parts of Queensland, Western Australia and northern NSW.3,7,8 Although sporotrichosis is readily treatable with oral anti-fungal medication, diagnosis is often difficult, and inappropriate antibiotic administration is common.1 Prompt diagnosis and management to reduce morbidity requires a high index of clinical suspicion.9 Clues that aid diagnosis include occupational exposure (laboratory personnel, veterinarians, miners, farmers), high-risk activities (including gardening, fishing or hunting), inoculating events (including bites or scratches by animals or insects) and the characteristic appearance of skin lesions.1 The purpose of this article is to describe an outbreak of sporotrichosis in New South Wales. This outbreak was investigated under the requirements of the New South Wales Public Health Act 2010 and no ethical approval was required.
Case
A teenage girl living in an outer Sydney suburb developed non-healing skin lesions on both knees after returning from a visit to a friend’s property on the New South Wales mid-north coast during the Easter school break in 2013. During this time she had spent a number of hours gardening, applying large quantities of mulch without wearing protective clothing on her arms or legs. Initially, the lesions resembled mosquito bites and later became purulent. The local doctor prescribed repeated courses of oral antibiotics but there was no improvement. A routine bacterial skin swab was negative. She was then referred to a dermatologist who performed a skin biopsy, which confirmed Sporothrix schenckii infection. Antifungal medication was commenced about 5 months after the initial development of symptoms. Her mother mentioned that other residents from the same town and surrounding area reported similar skin lesions.
 |
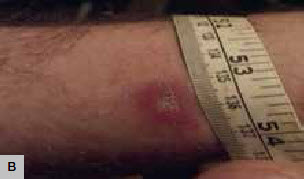 |
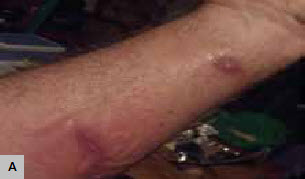
Figure 1. Erythematous lesions
A. Erythematous lesions of S.schenckii tracking along dermal lymphatics showing a characteristic ‘sporotrichoid spread’
B. Sporothrix lesion in a culture positive case |
The Population Health Unit attempted to identify all confirmed and probable cases of sporotrichosis using snowball sampling. General practitioners (GPs) in the town where the Sydney teenager had visited and its surrounding area were contacted to provide information on patients with confirmed or suspected diagnosis of sporotrichosis based on typical clinical presentation and/or laboratory confirmation during the first 6 months of 2013. Six cases of sporotrichosis were identified (including the index case). Two people had a positive diagnosis confirmed by culture. The remaining four people had strong epidemiological and clinical grounds to suspect sporotrichosis. Telephone interviews were conducted with all identified patients. During the interviews, detailed information was collected about the nature of their infection and possible exposures.
Representatives from the Department of Primary Industries (DPI) and Hunter New England Health conducted a joint inspection of the local farm that supplied hay to the residents. The aim of the inspection was to assess farming practices regarding procurement, storage and distribution of hay, and to collect samples for testing.
All those who were infected reported being previously healthy and had no background illnesses. All were local residents except for the Sydney teenager. All cases were exposed to the same batch of hay obtained from a local farmer between March and May 2013. Table 1 summarises patient demographics, clinical presentations and treatment for all cases. Four people (A, C, D, F) described contact with hay while mulching their gardens and two (B, E) while mulching an organic garlic farm. All except one (A) noted an offensive odour from the mulch. All developed skin lesions 2–8 weeks after the initial exposure (Figure 1a, b). None of the individuals recalled pre-existing skin abrasions.
Table 1. Sporotrichosis in mid-north coast of NSW, March–May 2013: patient demographics, clinical presentation and treatment outcome
|
|
Patient
|
Age
|
Sex
|
Area of residence
|
Occupation
|
Suspected exposure
|
Site of lesion
|
|---|
|
A
|
14
|
F
|
Sydney
|
Student
|
Garden mulch (friend’s property in Bobin)
|
B/L lower extremity multiple lesions
|
|
B
|
49
|
M
|
NSW mid-north coast
|
Farmer
|
Commercial garlic farming
|
B/L upper extremities multiple lesions; one on abdomen
|
|
C
|
52
|
F
|
NSW mid-north coast
|
Unemployed
|
Garden mulch
|
Right lower extremity – single lesion
|
|
D
|
61
|
F
|
NSW mid-north coast
|
School
administrator
|
Garden mulch
|
B/L upper extremities, multiple
|
|
E
|
22
|
M
|
NSW mid-north coast
|
Teacher
|
Helping case B on garlic farm
|
Face – single lesion
|
|
F
|
30
|
F
|
NSW mid-north coast
|
Stay-at-home
mum
|
Garden mulch
|
Right upper extremity – single lesion
|
|
#Culture result: Positive for S. schenckii; Negative for S. schenckii; Alt, Acremonium species positive, B/L, bilateral; N/A, sample not taken;
ICZ, itraconazole; H, heat therapy
†Microscopy positive
|
Table 1. Sporotrichosis in mid-north coast of NSW, March–May 2013: patient demographics, clinical presentation and treatment outcome
|
|
Onset month, 2013
|
Pre-existing
lesions
|
Swab
|
Biopsy
|
Culture result#
|
Treatment
|
Patient reported outcome
|
|---|
|
April
|
Nil
|
Yes
|
Yes
|
Positive
|
ICZ
|
Improved
|
|
May
|
Nil
|
Yes
|
Yes
|
Positive
|
H
|
Improved
|
|
April
|
Nil
|
Yes
|
No
|
Negative
|
H
|
Cured
|
|
March
|
Nil
|
Yes
|
No
|
Alt
|
H
|
Improved
|
|
May
|
Nil
|
No
|
No (skin scrapings)
|
Negative†
|
ICZ
|
Improved
|
|
April/May
|
Nil
|
No
|
Yes
|
N/A
|
H
|
Cured
|
|
#Culture result: Positive for S. schenckii; Negative for S. schenckii; Alt, Acremonium species positive; B/L, bilateral; N/A, sample not taken
ICZ, itraconazole; H, heat therapy
†Microscopy positive
|
 |
 |
Figure 2. Suspect hay bales
A. Bales of hay exposed to adverse weather conditions favourable for S.schenckii, on inspection of implicated farmer’s property
B. Inspection of implicated farmer’s property undertaken by Hunter New England Health and DPI Moist under surface of hay appears black and mouldy |
All cases were prescribed antibiotics (oral or topical) and four of the six patients were prescribed repeated courses of oral antibiotics. As there was a lack of clinical response, three of the six individuals (A, B, E) underwent skin biopsy and two (A, B) had positive culture results for S. schenckii. Patient E had a positive microscopy result. Patient D had skin scrapings sent for fungal examination, which was negative for S. schenckii but positive for Acremonium species. Patient C had a simple bacterial swab, which was negative, and Patient F had no samples taken for microbiological analysis.
The presumptive diagnosis was made in view of other confirmed positive cases from the same local area (in an endemic setting) within an overlapping time frame and following no response to repeated antibiotic treatment. The time delay between symptom onset and positive diagnosis was 5 months for the first confirmed case (A) and 3 months for the second (B).
Three of the six individuals (C, E, F) had single cutaneous lesions, whereas the remaining three had evidence of lymphocutaneous spread (Figure 1a). Only two people (one confirmed and one suspected) took oral antifungal treatment (itraconazole) and improved. The second confirmed patient (B) refused oral antifungal medication and self-treated with heat therapy. The remaining three suspected cases (C, D, F) also self-treated with heat therapy. All reported a very good response to heat treatment.
The farmer who supplied hay to all of the individuals cultivated his own produce. Bales were stored in the open (Figure 2a, b) and no personal protection was observed while handling the hay. Education was provided to the farmer regarding the risk of disease and its prevention. Samples were collected and sent for analysis but S. schenckii was not isolated from these samples. It is worth adding here that samples were obtained 6 months after the first cluster cases were identified and most of the hay had already undergone decomposition. Moreover, once the farmer was confronted by the angered community members and made aware of the problem he apparently burnt the implicated bales of hay.
Discussion
S. schenckii was first isolated in 1896 in Baltimore, Maryland.1 The first case in Australia was reported in 1951 in an elderly man following exposure while gardening.10
A study from the New South Wales mid-north coast area reported 31 cases of cutaneous sporotrichosis between 2000 and 2010.3 A Queensland study reported 16 cases over a period of 9 months,8 whereas a study from Western Australia reported a cluster of 11 cases between 2003–2004.7 All cases were exposed to mouldy or contaminated hay.
Sporotrichosis is classified into cutaneous, mucosal and extracutaneous forms.1 Cutaneous sporotrichosis usually follows minor skin trauma and presents as single or few lesions at the inoculation site after an incubation period of up to 3 months.11 A history of trauma may, however, be absent.12–14 The initial presentation is a papule or pustule, which subsequently ulcerates with a purulent discharge.1 Infection may spread via regional lymphatics giving rise to secondary lesions.1 However, lymph node involvement and systemic symptoms are uncommon.1
The disseminated cutaneous form presents with multiple skin lesions at non-contiguous sites, usually as a result of multiple inoculations.1 Spontaneous regression may occur in some cases.1
Rarely, nasal/conjunctival involvement can result from self-inoculation, haematogenous spread or fungal inhalation.15–18 Extracutaneous sporotrichosis can affect bones, joints and lungs. Although rare, this is more common in immunosuppressed individuals.19–21 Bony lesions may mimic osteomyelitis,1 and pulmonary lesions are similar to tuberculosis.22,23 Fortunately, systemic infection is extremely rare.1
Sporotrichosis is diagnosed on the basis of clinical, epidemiological and laboratory findings.1 Specimens include tissue biopsy and pus from skin lesions, and sputum, urine, blood, cerebrospinal fluid or synovial fluid for disseminated infections, depending on the site of infection.1 Samples are potentially infectious and must be labelled appropriately.7 Fungal cultures should be specifically requested.1 Specimens should never be placed in formalin as it kills the fungus.24
S. schenckii is readily cultured on Sabouraud’s medium at 25°C, forming cream-coloured colonies that eventually turn black.1 Microscopically, conidia are seen in a characteristic rosette-like arrangement.1 Definitive diagnosis is established by subculturing these colonies on enriched medium at 37°C when the fungus undergoes transformation into yeast forms.1 Differential diagnoses include:
- cutaneous leishmaniasis
- non-infectious skin ulcers
- mycobacteriosis
- nocardiosis
- chromoblastomycosis
- cryptococcosis
- blastomycosis
- sarcoidosis
- lupus vulgaris
- tuberculosis
- scrofuloderma
- cat-scratch disease
- other cutaneous bacterial infections.1
Itraconazole is currently the drug of choice for treatment of sporotrichosis.25 The dose is 200 mg daily for cutaneous and lymphocutaneous lesions and 200 mg twice daily for non-responsive cases, from diagnosis to 2–4 weeks after lesions have resolved (usually a total of 3–6 months).25 Amphotericin B is used for initial treatment of pulmonary and disseminated sporotrichosis.25 Local heat therapy (42–43°C) can be used in pregnant and lactating women, and it is of interest that a number of cases in the current cluster reported successful application of this mode of treatment with clinical resolution and no recurrence within 6 months.25 Use of potassium iodide is now limited because of its adverse effects.25
To prevent infection, personal protection measures should be used. At-risk individuals should be encouraged to wear gloves (preferably puncture-resistant), long-sleeved clothing, boots and masks while farming, gardening or undertaking other high-risk activity. Pre-existing cuts or wounds should be covered up to minimise exposure to the fungus. Exposed body parts should be thoroughly washed (preferably with antifungal solution such as chlorhexidine) following contact with potentially contaminated material.5 Animals with suspected disease should be handled carefully to minimise the risk of scratches, bites or contact with draining lesions.6 Theoretically, the fungus could penetrate intact skin.5 Thus, personal protection and hygiene precautions must be strictly observed. Currently there is no vaccine available for sporotrichosis.
Conclusion
Sporotrichosis may present a diagnostic challenge especially when typical sporotrichoid spread is absent. Treating clinicians, particularly those in areas with a known higher incidence (such as the New South Wales mid-north coast) and also across Australia, should be aware of the clinical presentation and send appropriate specimens for typical or suspected non-healing lesions. Referral to a dermatologist or infectious diseases specialist should also be considered, to avoid preventable morbidity and unnecessary antibiotic therapy.
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.