We examined the likelihood of general practitioners (GPs) ordering at least one of three coeliac/gluten-associated tests (CGATs) at BEACH GP–patient encounters in Australia in April 2000–March 2014. These CGATs were endomysial antibody, anti-gliadin antibody and anti-tissue transglutaminase. We also reviewed the health problems (coded in ICPC-2 PLUS1) for which a CGAT was ordered.
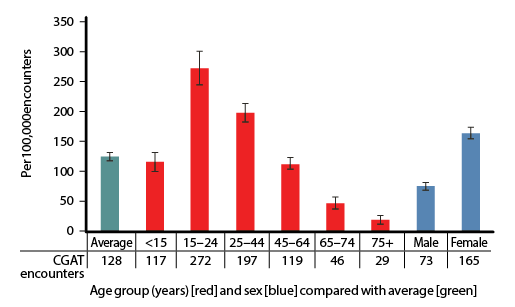 |
| Figure 1. Likelihood of coeliac/gluten associated test (CGAT) ordering per 100,000 encounters with patients in each age and sex group, compared with the average (95% confidence intervals; n = 1,372,100 encounters over 14 BEACH years, April 2000–March 2014) |
At least one CGAT was ordered at 1754 of 1,372,100 encounters (128 encounters per 100,000, 95% CI: 121–135). Figure 1 illustrates the age-specific and sex-specific likelihood of CGAT ordering per 100,000 encounters. The likelihood of CGAT ordering was significantly higher than average at encounters with patients aged 15–24 years and 25–44 years, and with female patients. The likelihood of CGAT ordering increased significantly over time, with more than a fivefold increase from 2000–01 to 2013–14 (Figure 2).
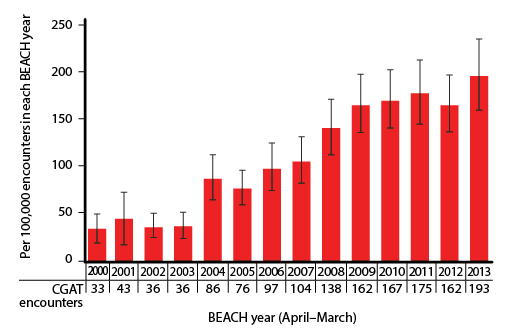 |
| Figure 2. Likelihood of coeliac/gluten associated test (CGAT) ordering per 100,000 encounters, for each BEACH year (95% confidence intervals; n = 1,372,100 encounters over 14 years) |
In BEACH, each recorded test is linked by the GP to one or more problems managed. At these 1754 encounters, 1841 CGATs were ordered and linked to 1885 problems. The top six problems (accounting for 41.1% of these links) were: irritable bowel syndrome, 9.5% (95% CI: 8.0–10.9); coeliac disease, 9.2% (7.8–10.7); abdominal pain, 8.9% (7.5–10.2); diarrhoea, 5.9% (4.8–7.1); iron deficiency, 4.5% (3.5–5.5); and bloating, 3.0% (2.2–3.8). Gluten intolerance accounted for only 0.8% (0.3–1.4) of links and gluten sensitivity for 0.3% (0.1–0.6).
Acknowledgements:
The authors thank the GP participants in the BEACH program, and all members of the BEACH team. Funding contributors to BEACH from April 2000 to March 2014: Australian Government Department of Health; Abbott Australasia Pty Ltd; AstraZeneca Pty Ltd (Australia); Bayer Australia Ltd; bioCSL (Australia) Pty Ltd; GlaxoSmithKline Australia Pty Ltd; Janssen-Cilag Pty Ltd; Merck, Sharp and Dohme (Australia) Pty Ltd; National Prescribing Service Ltd; Novartis Pharmaceuticals Australia Pty Ltd; Pfizer Australia Pty Ltd; Roche Products Pty Ltd; Sanofi-Aventis Australia Pty Ltd; and Wyeth Australia Pty Ltd. BEACH is approved by the Human Research Ethics Committee of the University of Sydney.