In this article we report changes in the GP management rate of psychological problems at encounters with patients aged 6–11 years (inclusive), annually over 13 years (April 2000–March 2013). These problems include all psychological conditions (psychological chapter of ICPC-2)2 and the five most commonly managed in 2008–2009 in this age group (anxiety, ADHD, intellectual impairment, behaviour disorder and ASD).1 At the 40 866 encounters with patients aged 6–11 years, 1438 psychological problems were managed, a rate of 35.2 psychological problems per 1000 encounters.
Comparing 2000–2001 with 2012–2013, there were significant increases in the annual management rate for ‘all psychological problems’, anxiety and ASD (Figure 1).
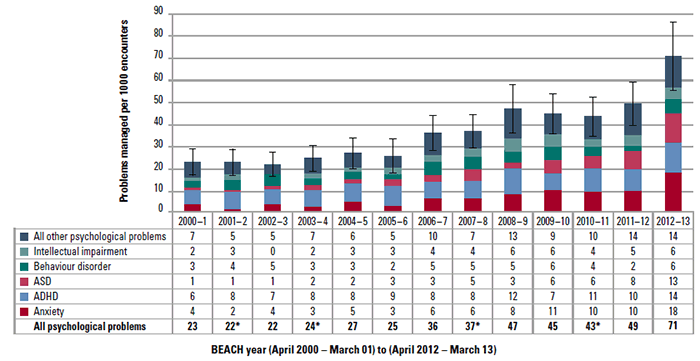
Figure 1. Annual number of psychological problems managed per 1000 GP– patient encounters with children aged 6 –11 years (95% CI for ‘All psychological problems’)
*Apparent discrepancies in totals are due to rounding. ASD = autism spectrum disorder; ADHD = attention deficit hyperactivity disorder
This is in line with the worldwide increase in paediatric psychological problems, which was noted and discussed in the 2011 paper.1 This paper also speculates that reduction in Better Access GP rebates introduced in November 2011 might have a negative effect on GP participation. However, this was not the case for encounters with patients aged 6–11 years where the management rate of ‘all psychological problems’ significantly increased between 2010–2011 (before changes) and 2012–2013 (after changes).
Acknowledgements
The authors thank the GP participants in the BEACH program, and all members of the BEACH team. Funding contributors to BEACH from April 2000 to March 2013: Australian Government Department of Health and Ageing; AstraZeneca Pty Ltd (Australia); CSL Biotherapies Pty Ltd; Merck, Sharp and Dohme (Australia) Pty Ltd; National Prescribing Service; Novartis Pharmaceuticals Australia Pty Ltd; Pfizer Australia Pty Ltd; Abbott Australasia Pty Ltd; Janssen-Cilag Pty Ltd; Sanofi-Aventis Australia Pty Ltd; GlaxoSmithKline Australia Pty Ltd; Bayer Australia Ltd; Wyeth Australia Pty Ltd; and Roche Products Pty Ltd. BEACH is approved by the Human Research Ethics Committee of the University of Sydney.