A high index of suspicion is required for the diagnosis of neuropathic pain as it can develop slowly over time. If neuropathic pain is suspected, a validated diagnostic screening tool such as the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS), the Self reported LANSS (S-LANSS), the Neuropathic Pain Questionnaire (NPQ), the Douleur Neuropathique en 4 (DN 4) questions, painDETECT and ID-Pain2 may be useful (see Resources). These verbal reports provide valuable information to the practitioner regarding pain quality: neuropathic pain is usually described as burning, painful, cold or electric shocks and may be associated with tingling, pins and needles, numbness or itching. These screening tools also serve as a good clinical record for follow up post-treatment initiation. They are not onerous to use and can be administered by the practice nurse or completed by the patient before the consultation. Pain will affect function, sleep, mood, work, and family and social life, therefore it is important to assess these as well as beliefs about the pain and self-management strategies (both helpful and unhelpful), as they will influence the pain and its management. The diagnostic algorithm in Figure 1 is a useful tool to help determine if neuropathic pain is likely.1
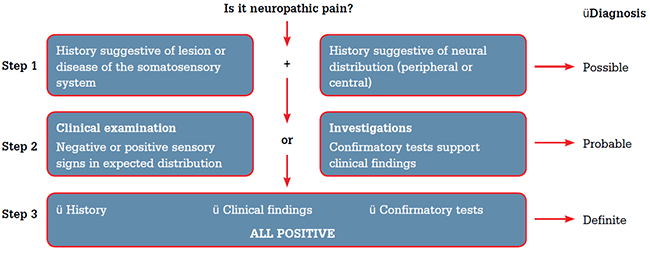
Figure 1. Assessing neuropathic pain
Step 1. A clinical history of disease or lesion of the somatosensory system suggests a possible diagnosis of neuropathic pain
Step 2. Confirmation by either clinically reproducible signs or investigations would suggest a probable diagnosis of neuropathic pain
Step 3. If the history, clinical examination and investigations are positive, this would support a definite diagnosis of neuropathic pain
Adapted from Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70:1630–5
Sensory examination includes response to light touch, temperature, painful stimulus, vibration and proprioception. Compare both sides and grade as normal, decreased or increased. Motor testing includes tone, strength, reflexes and coordination. Also look for autonomic changes in colour, temperature, sweating and swelling.
Imaging may be required to exclude nerve entrapment and disc pathology, usually with computed tomography (CT) or magnetic resonance imaging (MRI). Nerve conduction studies and electromyography are useful if large myelinated axonal damage is suspected. Routine blood tests to exclude differential diagnoses include full blood count (FBC), erythrocyte sedimentation rate (ESR), glucose, creatinine, alanine transaminase (ALT), vitamin B12, serum protein immunoelectrophoresis and thyroid function. Assessing glycaemic control with an HbA1c is useful in patients who are diabetic. A glucose tolerance test may be helpful if diabetic status is not known.
Current evidence-based recommended treatments for neuropathic pain conditions are outlined in Table 1; medications and suggested treatment doses are outlined in Table 2.
Table 1. Current evidence-based recommended treatments for neuropathic pain conditions2,5,16–19
| Drug therapy | Diabetic polyneuropathy | Postherpetic neuralgia | Trigeminal neuralgia | Chronic regional pain syndrome# |
|---|
| First line |
Duloxetine
Gabapentin
Pregabalin
TCAs
Venlafaxine |
Tricyclic antidepressants (TCAs)
Gabapentin
Pregabalin
5% lignocaine patch |
Carbamazepine
Oxycarbazepine |
Oral prednisone
Bisphosphonates
Gabapentin
Opioids
NSAIDs
Topical capsaicin
lV lignocaine |
| Second or third line |
Tramadol
Opioids* |
Opioids
Tramadol |
Baclofen
Lamotrigine |
| Others |
|
|
|
Anti-TNF antibodies
Calcitonin |
* Third line therapy because of the risks associated with long-term use (tolerance, addiction, misuse)
# CRPS does not fit the current IASP criteria for neuropathic pain. Current evidence suggests that the pathophysiology is multifactorial (neurogenic inflammation, central and peripheral neuroplasticity) |
Diabetic polyneuropathy
It is estimated that 3.6% of Australians have diabetes and about 10% of patients (10% males, 9.4% females) with newly diagnosed diabetes were reported to have diabetic polyneuropathy (DPN),3 while another study reported between 16% and 49% of patients with newly diagnosed diabetes.4 It is not currently possible to predict which patients will develop DPN, although the current research tools, such as quantitative sensory testing and laser evoked potentials, may be of benefit in the future as they can detect minor nerve damage.
Table 2. Medications and suggested doses for treating neuropathic pain21
| Drug* | Recommended dose | Side effects | Considerations |
|---|
| Gabapentin |
300–1200 mg three times daily20 |
Sedation, dizziness, headache, oedema, weight gain, ataxia, tremor, nystagmus, anorexia, asthenia |
Pharmacokinetics variable, so dose is individual-dependent
Reduce dose in renal impairment
Not available on the PBS |
| Pregabalin |
50–300 mg twice daily |
Pharmacokinetics linear and more predictable
Announced will become available on the PBS (details pending) |
| Carbamazepine |
100–600 mg twice daily |
Evidence only for trigeminal neuralgia |
| Oxycarbazepine |
300–1200 mg twice daily |
Plasma levels |
| Tricyclic antidepressants |
10–75 mg at night |
Cardiac arrhythmia, dry mouth, sweating, dizziness, blurred vision, sedation, constipation, urinary retention, cognitive disturbance |
Baseline electrocardiogram |
| Duloxetine |
30–60 mg/day |
Nausea, somnolence, dry mouth, constipation, diarrhoea, hyperhidrosis, dizziness
May elevate blood sugar levels, liver function tests, blood pressure |
Precaution in severe hepatic dysfunction, unstable hypertension
Titrate to effect and side effects |
| Venlafaxine |
150–225 mg/day |
Opioids
- morphine
- oxycodone
- hydromorphone
- fentanyl
- buprenorphine
|
Start 5 mg/day and titrate dose |
Constipation, sedation, nausea, dizziness
Can affect the endocrinological and immunological systems |
No evidence for long-term use
Regular assessment is useful using the 5As approach of: Analgesia, Activity, Adverse effects, Aberrant use, Affect |
| Tramadol |
50–400 mg/day |
Dizziness, dry mouth, nausea, constipation, somnolence, cognitive impairment |
Lowers seizure threshold
Serotonin syndrome |
| Topical capsaicin cream |
0.025–0.075% four times daily |
Burning sensation |
|
| 5% lignocaine patch |
Apply to affected area for 12 hours per day (trigeminal neuralgia) |
Local effects only (eg. mild rash) |
Low systemic absorption
Not available on the PBS |
| * A combination of therapies using different classes is sometimes beneficial and could be tried (eg. an opioid and TCA) |
Diabetic polyneuropathy affects both the autonomic and peripheral nervous system. Peripheral nerve involvement generally presents as tingling or burning in the hands and feet, and patients may report paroxysmal ‘electric shocks’, an increased sensitivity to painful stimuli (hyperalgesia) or pain to non-painful stimuli (allodynia). Unawareness of injury, ulcers and infection in the affected limb may occur. The pain often interferes with daily activities and sleep. Clinical features include reduced sensation to light touch and vibration, with reduced ankle jerks and mild weakness. Typical pattern of sensory loss is in a ‘glove and stocking’ distribution which may extend proximally with disease progression. Injury, ulcers or infection may be seen on examination. Most commonly patients present with burning feet that disturbs their sleep. On examination there may be trophic changes or evidence of injury that has gone unnoticed due to the loss of sensation.
Rare episodes of painful DPN have been reported with acute ketoacidosis and even in patients with tight glycaemic control. In these cases, the pain is nocturnal and is described as ‘burning’ and is exacerbated by light touch (eg. bedding or clothing). Examination reveals hyperalgesia, but little in the way of sensory or motor loss.
Differential diagnoses are extensive, including neurotoxicity (eg. from medications or heavy metals), nutritional deficiencies, alcohol related neuropathy, thyroid or electrolyte disorders, nerve entrapment, or inflammatory or congenital causes.
Management of diabetic polyneuropathy
Primary prevention with early diagnosis, exclusion of underlying causes and good glycaemic control, coupled with appropriate lifestyle changes, will delay progression and the development of complications. The current evidence for DPN supports the use of tricyclic antidepressants (TCAs), serotonin-norepinephrine reuptake inhibitors (SNRIs) (venlafaxine and duloxetine), gabapentin, pregabalin, tramadol, morphine and oxycodone.5 Appropriate footwear and podiatry care is also important.
Postherpetic neuralgia
Postherpetic neuralgia (PHN) is pain that lasts for more than 3 months after the onset of a herpes zoster infection. The pain intensity may be mild, moderate or severe; duration is highly variable with some case studies reporting pain several years after the initial infection.6 While the incidence of PHN is not known, it is uncommon in those aged less than 50 years. Some patients have recurrent episodes of herpes zoster without developing PHN.
Postherpetic neuralgia is a clinical diagnosis. The patient describes a sharp or burning pain or ache in a dermatomal distribution. The affected skin may be sensitive to light touch (eg. bedding or clothing) while some patients experience frequent excruciating paroxysmal bouts of shooting pain. Differential diagnoses to consider if the face is involved are cluster headaches, a peripheral nerve lesion, neurodermatitis or infection. Examination may reveal evidence of past vesicular rash in a dermatomal distribution and hyperalgesia or allodynia.
Management of postherpetic neuralgia
Management is divided into prevention and management of the acute herpes zoster infection and the more challenging task of treating the symptoms of PHN. Paediatric vaccination reduces varicella infection through IgG antibody formation. Adult vaccination (Zostavax®: ~$200, not listed on the Pharmaceutical Benefits Scheme) stimulates T-cell mediated immunity and may reduce the incidence of both herpes zoster and PHN.7 Early antiviral therapy is shown to reduce the severity and duration of an acute herpes zoster infection. Corticosteroids and oxycodone reduce the pain experienced during the acute infective period. Pregabalin, gabapentin, TCAs (amitriptyline and nortriptyline), controlled release opioids, capsaicin cream (Zostrix®) and 5% lignocaine patches (Versatis®) may also help reduce the pain of PHN.
Trigeminal neuralgia
Trigeminal neuralgia (Tic douloureux) (TGN) is characterised by sudden severe brief episodes of recurrent stabbing pain in the distribution of one or more branches of the fifth (V-trigeminal) cranial nerve.9 It is relatively rare and the majority of cases present unilaterally. Epidemiologically, it is twice as likely to affect women and is more common in the 50+ years age group.10 Trigeminal neuralgia can affect one, two or all three branches, but most cases present in the maxillary or mandibular branches with only 2% of cases affecting the ophthalmic branch.10
Symptoms of TGN include jaw pain that may be aggravated by chewing, swallowing, talking, touch,or by consuming hot or cold food and drink. The pain can be triggered by shaving or wind blowing across the face. The severe paroxysms of pain are often described as ‘shooting’, ‘sharp’ or ‘electric’. A pathognomonic feature is the presence of trigger zones in the distribution of the affected nerve. An attack has rapid onset and lasts between 10 seconds and a couple of minutes, followed by a refractory period. Weight loss, insomnia due to pain and reduced functioning may occur. Examination may reveal allodynia or hyperalgesia in the distribution of the affected nerve.
The generally accepted common cause is compression of the Gasserian ganglion (sensory ganglion of the trigeminal nerve) or its branches by a blood vessel. Differential diagnoses include multiple sclerosis, tumour, trauma or injury, trigeminal autonomic cephalagias, temporal arteritis, migraine or atypical facial pain. Thin-cut MRI is used diagnostically to exclude the need for surgical intervention.
The mainstay of pharmacological management is carbamazepine with a starting dose of 200–400 mg/day. Pain relief is seen in one of every two cases (number need to treat [NNT] 1.7–1.8).11 Oxycarbazepine can also be used. Baclofen has been reported as efficacious, however a Cochrane review concluded insufficient evidence to support it as a unimodal treatment for TGN.12 Gabapentin, pregabalin, topiramate and older anticonvulsants have also been used in refractory cases.
Surgical treatments are either decompressive or ablative. Microvascular decompression has the highest success rate with 76% probability of pain relief at 5 years with minimal complications. Other procedures to consider are radiofrequency or glycerol ablation (rhizotomy), balloon microcompression and gamma knife surgery, which has a 45% probability of pain relief at 5 years.13
Complex regional pain syndrome
Complex regional pain syndrome (CRPS) is rarely seen in general practice. Diagnosis is based on a cluster of clinical criteria affecting the somatosensory and autonomic nervous systems. However, CRPS remains a classification enigma: both neuropathic and other non-neuropathic pathophysiological processes have been suggested.
Early recognition in primary care, implementation of treatment and referral to a pain service will help minimise function loss, chronicity and disability.
A patient with CRPS typically presents with severe pain on movement, with skin colour and temperature changes, and sweating and swelling that occurs in a regional distribution. Reduced movement, weakness and tremor may also occur. Clinical signs include vasomotor and sudomotor (relating to sweat glands) changes, motor signs, pain, allodynia, hyperalgesia and reduced range of movement and strength. Later clinical signs include trophic changes (nails, skin, hair) and osteoporosis. In CRPS-I, recognised precipitating events include fractures, sprains or post-surgery. CRPS-II may develop after major peripheral nerve injury.
There is no specific test for CRPS. Plain X-rays (cortical thinning and bone loss), bone scans (abnormal third phase increased peri-articular uptake), temperature differences, quantitative sensory testing and MRIs are used both clinically and in research. However, only ‘objective measurement of temperature differences’ has a high sensitivity and specificity.14
Criteria for clinical diagnosis is continuing pain disproportionate to an inciting event, coupled with three of four symptoms plus at least one sign from the following: sensory, vasomotor, sudomotor, motor/trophic, and with no other diagnosis that better explains the patient’s symptoms and signs (Table 3).15
Table 3. The Budapest Diagnostic Criteria for diagnosis of complex regional pain syndrome
| Examination | Features | Symptoms | Signs |
|---|
| Vasomotor |
Skin colour/temperature asymmetry |
|
|
| Sudomotor |
Swelling/sweating (hypo- or hyper-hidrosis) |
|
|
| Motor/trophic |
Reduced movement/weakness/tremor/trophic changes |
|
|
| Pain |
Spontaneous pain: mechanical, thermal, deep somatic hyperalgesia |
|
|
| Score |
|
Three or more (+) |
One or more (+) |
Score (+) if symptom or sign is present, (–) if absent. Three positive symptoms and one positive sign is required for a considered diagnosis of CRPS
Adapted from Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med 2007;8:326–31 |
Differential diagnoses to consider are unilateral vascular disease, post-traumatic neuralgia, metabolic, autoimmune or neoplastic disorders, neuropathies or psychiatric somatoform disorders.
With only a few evidence-based clinical trials for treating CRPS, treatments are extrapolated from studies of other neuropathic conditions. An older, randomised double-blinded, placebo-controlled trial showed limited improvement with gabapentin.16 Less rigorous trials and case studies have shown some benefit using non-steroidal anti-inflammatory drugs (NSAIDs), opioids, baclofen, calcitonin, corticosteroids, bisphosphonates, dimethyl sulfoxide, IV immunoglobulin therapy and TCAs, while intravenous lignocaine temporarily reduces spontaneous evoked pain.17 Some benefit has been reported using anti-TNF antibodies (infliximab).15 Treatment options for complex CRPS are outlined in Table 4.
Table 4. Treatment options for complex regional pain syndrome17
| Some evidence | No evidence (worth considering) |
|---|
Bisphosphonates
Gabapentin
Corticosteroids
Topical 50% dimethyl sulfoxide
Anti-TNF antibodies (infliximab)15
IV immunoglobulin therapy |
NSAIDs
Opioids
TCAs
SNRIs
Sodium channel blockers |
There is little evidence for invasive procedures, particularly in the early treatment of CRPS. Sympathectomy does not provide lasting analgesia and may worsen the pain. Spinal cord stimulation produces short-term improvement in refractory cases and could be considered in combination with behavioural and physical therapies. Current treatment of CRPS is directed toward restoration of function using pharmacological, psychological and physical therapies. In practice, first line pharmaceutical agents to consider are opioids, antidepressants, gabapentinoids, carbamazepine and corticosteroids.
Diagnosis of CRPS is important, as early intervention reduces the severity of and functional disability associated with this condition. In CRPS-I, fractures are associated with a better prognosis than other causes. Unfortunately, it has been suggested that a majority of 60% of patients with CRPS-II have a prolonged course, even with intensive therapy.
Key points
- Neuropathic pain is usually described as burning, painful, cold or electric shocks and may be associated with tingling, pins and needles, numbness or itching.
- Screening tools are valuable in diagnosing neuropathic pain and also serve as a good clinical record for follow up.
- Imaging may be required to exclude nerve entrapment and disc pathology, usually with CT or MRI scanning.
- Diabetic polyneuropathy affects both the autonomic and peripheral nervous system. Management includes exclusion of underlying causes, good glycaemic control, appropriate lifestyle changes, and the use of gabapentinoids, TCAs, SNRIs and opiates.
- Postherpetic neuralgia is pain that lasts for more than 3 months after the onset of a herpes zoster infection. Management includes prevention with varicella vaccination and early antiviral therapy and treatment with gabapentinoids, TCAs, controlled release opioids, capsaicin cream and lignocaine patches.
- Trigeminal neuralgia is characterised by sudden severe brief episodes of recurrent stabbing pain in the distribution of one or more branches of the fifth (V-trigeminal) cranial nerve. Carbamazepine is first line treatment.
- Diagnosis of complex regional pain syndrome is based on the Budapest Diagnostic Criteria.
Resources
Competing interests: Milana Votrubec has received payments from Mundipharma and Janssen-Cilag for chairing pain medicine meetings and from Mundipharma, Pfizer and Janssen-Cilag for workshop development. Ian Thong has received payments from Mundipharma for GP talks.
Provenance and peer review: Commissioned; externally peer reviewed.