Women who have ever had a biopsy-confirmed cervical HSIL (histological evidence of cervical intraepithelial neoplasia (CIN) 2 or 3, moderate or severe dysplasia, or carcinoma in-situ) are eligible for the recommended testing regimen. This includes women with HSILs at any time in the past, whether 1 year or over 20 years ago.
Human papillomavirus and cervical changes
It is now well established that the primary cause of cervical cancer is persistent infection with one of the high risk or oncogenic HPV genotypes.2
There are approximately 200 different types of HPV, which are classified according to DNA sequence. Between 40–50 HPV types specifically infect the anogenital area and, less commonly, the oro-pharyngeal area. These HPV types primarily spread through genital skin-to-skin contact.
These genital HPV types are divided into ‘low risk’ and ‘high risk’ according to their association with, and ability to cause, anogenital cancer. Low risk types include types 6 and 11, which are responsible for approximately 90% of genital warts. These types do not cause anogenital cancer.
There are 15 high risk or oncogenic HPV types: HPV 16 and 18 are the two most important types, and are responsible for around 70% of invasive cervical cancers and 50% of high grade lesions.2 Tests to detect the high risk HPV types have been available in Australia for many years.
Infection with HPV is common and mostly transient. Most cervical HPV infections (including the high risk types 16 and 18) are cleared or suppressed by cell mediated immunity within 1–2 years of exposure.2
Human papillomavirus genital transmission
Human papillomavirus is transmitted to the genital area when an infected partner sheds virus, which enters the genital skin through micro-abrasions in the epithelium. The virus is contained within the epithelium and does not cause viraemia. Typically, HPV enters the basal cells and uses the host cell replicative enzymes to assemble and release new virions from the uppermost cells of the squamous epithelium.3
In some cases, associated with the high risk HPV types, the HPV genome becomes integrated into the host chromosome. In this form, the infection is more likely to become persistent and cellular changes occur that may be recognised as ‘high grade’ changes on a Pap smear.
High grade squamous intraepithelial lesions
Cervical cytology (the Pap smear) examines exfoliated cells from the cervix to detect significant cellular changes associated with persistent HPV infection. Persistent infection with one of the high risk HPV types may be seen as ‘high grade’ changes or HSILs. These changes include features such as a dense or double nucleus, or a high nuclear-cytoplasmic ratio.
High grade changes are reported at a rate of around 8.5 per 1000 cervical cytology tests.4 This means that around 21 000 Australian women receive a report of an HSIL each year.
Data from Australian laboratories suggest that women with HSIL cytology reports have a 62–89% chance of harbouring actual high grade cervical intraepithelial disease and a 0–3% chance of having an invasive cervical cancer.2 Therefore, under the NHMRC guidelines women with a possible or definite HSIL detected on cervical cytology should be referred for colposcopy.1
A review of the literature on the natural history of CIN has shown that women with histological evidence of CIN 2 have a 5% chance of progression to cervical cancer, and women with histological CIN 3 have a 12% chance of progression.5 It is therefore recommended that these women be treated in order to reduce their risk of developing invasive squamous cell cervical cancer. Treatment may be ablative or excisional.
Development of cervical cancer
There are four main steps for the development of cervical cancer:2
- infection of the metaplastic epithelium at the cervical transformation zone (the squamo-columnar junction) with a high risk or oncogenic type of HPV
- persistent infection with an oncogenic HPV type
- progression of persistently infected epithelium to pre-cancerous changes (HSILs), typically taking 1–15 years to become established
- invasion through the basement membrane of the epithelium. Invasive cervical cancer develops over many years. Rapid onset cancers can occur, but they are very rare.
Human papillomavirus testing post-HSIL treatment
Women treated for a biopsy-confirmed HSIL should return to their specialist for repeat colposcopy and cervical cytology 4–6 months after treatment. If these tests are satisfactory, there is no reason why the woman should not return to her usual general practitioner for subsequent Pap tests and HPV testing.
Testing for high risk HPV types is an important part of the management of women who have been treated for HSIL. Several studies have shown that these women are at increased risk of further high grade disease.6–11 After treatment, the vast majority of women will clear their oncogenic HPV infection within 24 months (Figure 1).12–18 The reliable negative predictive value of HPV testing allows these women to return to the routine Pap screening interval.
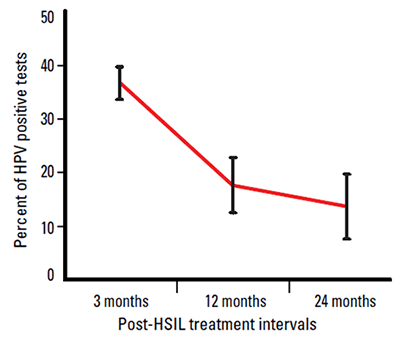
Figure 1. HPV infection rates by time since HSIL treatment
When to perform HPV testing
Cervical cytology and HPV testing should commence 12 months after treatment of a biopsy-confirmed HSIL and continue annually until the woman has tested negative for both tests on two consecutive occasions. At this point the woman can then return to regular population screening, according to the recommendations for the general female population, which is currently two-yearly (Table 1). Medicare covers two HPV tests in these circumstances in an ongoing 2 year period (MBS Item number 69418).
Table 1. HPV testing recommendations post-HSIL treatment
| Time since treatment | Cervical cytology | Coloposcopy | HPV testing |
|---|
| 4–6 months |
√ |
√ |
|
| 12 months |
√ |
– |
√ |
| 24 months |
√ |
– |
√ |
Continue cervical cytology and HPV testing every 12 months until these tests are both negative on two consecutive occasions
The Medicare rebate for HPV testing in these circumstances covers two HPV tests in an ongoing 24 month period (item number 69418) |
Human papillomavirus testing beyond these recommendations is not currently advised (Table 2).
Table 2. Current NHMRC guidelines for when HPV testing is not recommended1
|
Less than 12 months after treatment of a high grade cervical lesion
|
|
As a screening test
|
|
As a triage tool for women with low grade squamous intra-epithelial cervical lesions (unless this is part of the follow up post-treatment of an HSIL)
|
If the GP is uncertain of a patient’s medical history, state cervical cytology registries can usually provide further information to guide subsequent testing.
The frequency of positive HPV tests following treatment for a high grade lesion is high in the first 12 months after treatment, but diminishes significantly after 12 months, as shown in Figure 1. Figure 1 also demonstrates the importance of not testing too soon after treatment.
If HPV testing is performed outside the NHMRC guidelines, pathology laboratories will usually charge about $60–100.
How to perform HPV testing
HPV testing is usually performed by taking a swab or brush sample from the endocervix after cervical cytology has been taken. This is then placed in a transport tube (provided by your laboratory) for testing. The resources listed at the end of this article provide further details on sample collection.
If a liquid based sample is being collected for other reasons, HPV testing can be done on this sample.
Different methods for testing for oncogenic HPV types are available; check with your laboratory for their requirements. It is extremely important to make sure that the test offered has been clinically validated.
The result should be available at the same time as the cervical cytology report.
Clinical scenarios
Jenna, 57 years of age, had treatment for a biopsy-confirmed HSIL in 1989. Since then she has returned conscientiously every 12 months for a Pap test as she was originally told to do. Her smear results have been negative since treatment. What will your practice be when you next see her for her annual Pap test?
You can explain to Jenna that there is now a reliable test to check for the presence of the HPV types that can cause cervical cancer. It is a very sensitive and accurate test. The vast majority of women, you explain to her, clear this virus within a year or two of treatment. If, even after such a long time, this is found to be the case with her, on two consecutive occasions 12 months apart, she will be able to return to 2-yearly instead of annual Pap tests. In these circumstances, the test is eligible for the Medicare rebate.
Emily, 28 years of age, is seeing you 6 months after she was reviewed by her gynaecologist post-treatment of a high grade cervical lesion (CIN 2) 12 months ago. The letter from the gynaecologist assures you that the Pap smear and colposcopy at 6 months were both normal. Emily is new to your practice and has a few questions about HPV that she feels have not been adequately answered.
You explain to Emily that the current recommendation is that she have a Pap test and an HPV test (to check for viral clearance) every 12 months until she has a negative Pap test and a negative HPV test one year, followed by a negative Pap test and a negative HPV test the following year. After that she can return to the two-yearly interval.
The results show a negative Pap smear and a positive HPV test. You can tell Emily that the most likely explanation for this is that her body is still clearing the virus. This process can take a couple of years. She needs a repeat Pap test and HPV testing in 12 months.
Martha, 43 years of age, presents to you for a Pap test. She has recently moved from interstate. She recalls that around 1998 she had a smear that showed some ‘abnormal cells’, and that she had treatment for these at that time. She has no idea what the biopsy actually showed. She has had two or three smears since then, which she believes were negative. What should you do?
Your state cervical cytology registry will be able to provide the telephone number for other state cervical cytology registries. It is confirmed that in May 1997, Martha had a cervical biopsy that showed a mild dysplasia (CIN 1); she has had three smears since then, all of which have been negative.
As Martha did not have a biopsy-confirmed high grade lesion she is not eligible for Medicare rebatable HPV testing. Before the NHMRC guidelines (2005), low grade (CIN 1; mild dysplasia) lesions were often treated. Today you just need to do a Pap smear on Martha and remind her of the importance of regular routine cervical screening.
Sally, 66 years of age, has a past history of a biopsy-confirmed high grade lesion when she was aged 36 years (in 1983). She has presented reasonably regularly over the years, and apart from a low grade smear (possible HPV) in 1993, all her other annual smears have been negative, including a Pap test and HPV test 12 months ago. Today you take another Pap smear and an HPV test. The HPV test is negative, the Pap smear is also negative but has no endocervical cells present.
On reviewing your records for Sally, you note that you have stated that you saw the cervical os clearly and you were able to insert both the cytobrush and the swab/brush for the HPV test into it. If this is the case, the Pap smear does not have to be repeated. We know that about 12% of women over the age of 60 years have a transformation zone that is presumably higher than the length of the cytobrush.19 It is not recommended that the brush is ever inserted such that the lower bristles cannot be seen. Sally can now return to two-yearly screening until the age of 70 years, at which point, should her intervening smears continue to be negative, she can cease having Pap tests.
Lesley, 38 years of age, had a high grade cervical lesion treated just under 2 years ago. She had her first Pap test and HPV test 12 months post-treatment: both tests were negative. She knows she has come in a little early for her repeat test (11 months after the last tests), but she is about to go overseas for 6 months and is keen to have the tests done before she goes. What will you do?
The first Pap test and HPV DNA tests must be no earlier than 12 months after the high grade histology result, but the time between further tests and any preceding test of each type (smear, HPV DNA) has to be at least 10 months.20 Therefore it is quite clinically acceptable to repeat Lesley’s tests at this time rather than have her wait until her return from overseas.
Summary
Oncogenic HPV testing is a reliable and extremely sensitive test that is Medicare rebatable when used in the management of women who have ever had a histologically-confirmed HSIL treated. The appropriate use of testing for the high risk, oncogenic, HPV types is a valuable tool, as the vast majority of women in these circumstances will become negative for these oncogenic types. This will not only be reassuring for these women, but will bring them back into the routine cervical screening interval.
Our challenge is to encourage its use.
Resources
- Taking a Pap test: instructional DVD. Produced by Victorian Cytology Service and Melbourne Sexual Health Centre, and supported by PapScreen Victoria. This DVD demonstrates how to take an HPV test (as well as how to take a Pap smear). It is available free of charge from VCS Pathology by calling 03 9250 0300. It can also be watched online at www.mshc.org.au. Go to ‘health professionals’ then ‘videos’
- Cervix sampling card. This A4-sized laminated card shows pictures of different cervices, and describes the recommended techniques and instruments that should be used when taking a Pap test. It also describes testing for HPV using the Hybrid Capture brush. The card is free of charge and is available from PapScreen Victoria at www.papscreen.org.au
- HPV: A guide for practitioners. This A4-sized laminated sheet has a check-list for explaining HPV to patients, as well as the answers to many common questions patients ask. It also gives some background on HPV for medical practitioners, explains the purpose of HPV testing, outlines HPV testing post-HSIL treatment, and provides common scenarios with differing results for patients who have been treated for an HSIL lesion. It is free of charge from PapScreen Victoria at www.papscreen.org.au.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.