What is FibroScan® and transient elastography?
FibroScan® is a non-invasive device that assesses the ‘hardness’ (or stiffness) of the liver via the technique of transient elastography. Liver hardness is evaluated by measuring the velocity of a vibration wave (also called a ‘shear wave’) generated on the skin. Shear wave velocity is determined by measuring the time the vibration wave takes to travel to a particular depth inside the liver.1 A graphical representation of this is provided on the screen (Figure 1). Because fibrous tissue is harder than normal liver, the degree of hepatic fibrosis can be inferred from the liver hardness. To improve test reliability a minimum of 10 valid readings, with at least a 60% success rate and an interquartile range of ≤30% of the median value, are taken with the results expressed in kilopascals (kPa).1,2
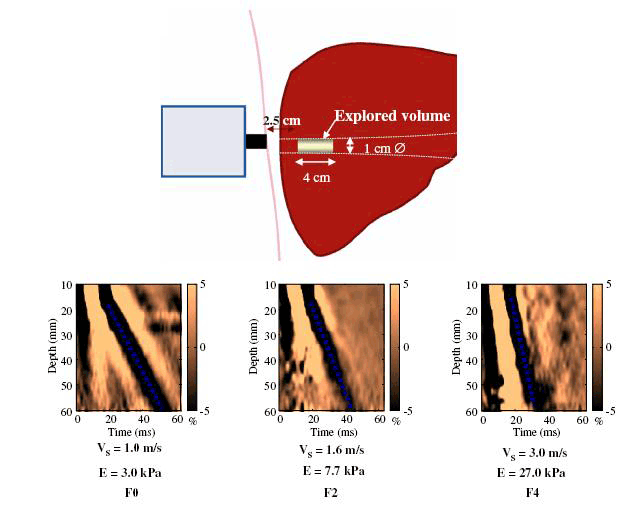
Figure 1. Examples of liver stiffness measurements
V = velocity, E = Elastic modulus, and is calculated as E=3xV3
Reproduced from Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835–47 with permission from Elsevier
How is the test performed?
With the patient lying supine, an ultrasound-like probe is placed on the skin over the liver area, typically in the right mid-axillary line. The patient feels a gentle ‘flick’ each time a vibration wave is generated by the probe (Figure 2). Typically the test takes around 10 minutes to perform and causes no patient discomfort. In general, patients should have fasted for at least 2 hours before the procedure, although specific instructions may vary according to the operator.
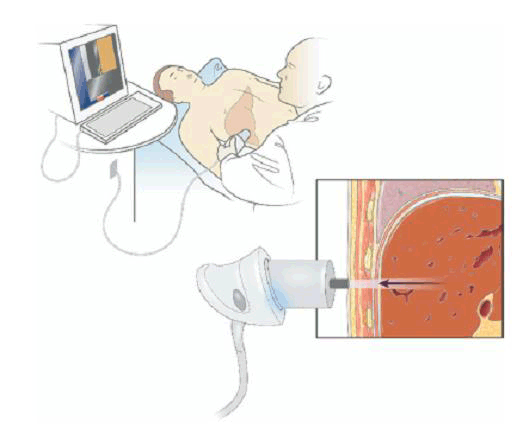
Figure 2. An example of the technique
Reproduced from Rockey DC. Noninvasive assessment of liver fibrosis and portal hypertension with transient elastography. Gastroenterology 2008;134:8–14 with permission from Elsevier
What are the indications for this test?
FibroScan® is principally used to estimate the degree of liver scarring present (ie. stage of liver disease). This is very useful in the assessment of patients with chronic liver disease, including chronic hepatitis C, chronic hepatitis B, chronic alcohol abuse and fatty liver. The concept is that as more fibrosis and scarring occur, the higher the liver stiffness reading will be. This reading may be used to:
- estimate the existing degree of liver damage
- monitor disease progression or regression via serial measurements
- guide prognosis and further management, including treatment.
What are the pitfalls of this test?
Transient elastography does not directly measure fibrosis and hence, false elevations (ie. over-estimation of liver fibrosis) may be observed for several reasons (Table 1). Therefore, liver stiffness readings need to be interpreted carefully, and with consideration to these potential confounding factors.
Table 1. Possible reasons for an over-estimation of fibrosis using FibroScan®
- Liver inflammation (eg. active hepatitis)
- Cholestasis (eg. biliary obstruction)
- Mass lesions within the liver (eg. tumour)
- Liver congestion (eg. heart failure)
|
In addition, there are some patients in whom accurate readings cannot be obtained (Table 2).
Table 2. Failure or unreliable readings are seen more frequently in patients with the following characteristics
- Obesity (BMI >30–35 kg/m2)
- Older age
- Presence of ascites
- Features of the metabolic syndrome (type 2 diabetes, hypertension, increased waist circumference)
|
This is because the ultrasound-based technique requires an adequate visualisation of the liver to obtain readings. In our experience, accurate readings are obtained in <50% of patients who have a body mass index (BMI) >35 kg/m2 using the standard M probe. This problem is partially overcome through the development of different probes (such as the XL probe), that allows deeper penetration of vibration wave.3 In addition, an S probe may be required to obtain readings from patients with very small rib spaces, or in children. One large study from Europe indicated that FibroScan® was unable to obtain accurate readings in about 20% of patients. Factors associated with reading failure or unreliability were BMI >30 kg/m2, increasing age, and features of the metabolic syndrome, among others.4
There are no absolute contraindications for the test, although the presence of ascites prevents propagation of the vibration wave and therefore frequently results in failure to obtain readings. The manufacturer also advises against the use of this device in pregnancy and in patients with a pacemaker.
How does it fit in with ultrasound?
FibroScan® only assesses liver stiffness and therefore does not replace conventional ultrasound. This is important to note because chronic liver disease patients usually require an ultrasound examination to assess the structural integrity of the liver, and to look for features of portal hypertension such as dilated portal vein, recanalisation of ligamentum teres, abdominal varices, and splenomegaly. In addition, conventional ultrasound should be performed at 6–12 monthly intervals to screen patients with cirrhosis for hepatocellular carcinoma. As transient elastography cannot perform these functions it is complementary to conventional ultrasound in the evaluation of liver disease.2
FibroScan® is superior to ultrasound for the detection of liver scarring and therefore may be used to determine if cirrhosis or advanced fibrosis is present at the initial assessment and whether it has developed during follow up because of disease progression. Furthermore, recent studies have shown that the liver stiffness provides prognostic information, particularly in subjects with chronic viral hepatitis B and C, including the risk of future liver decompensation,5 liver cancer6,7 and survival.8
How does it fit in with other tests for assessment of liver scarring?
More recently other non-invasive tools to assess liver scarring have been developed such as acoustic radiation force impulse (ARFI), real-time elastography (RT-E), magnetic resonance elastography (MRE), and shear wave elastography (SWE).2 These devices also use the physical properties of the liver to infer information about liver scarring. While their use is less widespread than FibroScan®, preliminary data suggests they provide similar information and diagnostic accuracy, with their use dependent upon local availability and expertise.2 Like FibroScan®, these techniques do not attract a Medicare rebate and therefore some centres will charge out-of-pocket expenses.
Aside from these devices that have the ability to assess an intrinsic physical property of the liver, there are a range of serum markers or biomarkers designed to identify patients with advanced fibrosis or cirrhosis. The aspartate aminotransferase/alanine aminotransferase (AST/ALT) ratio or AST to platelet ratio index (APRI) are freely available and can be calculated on routine blood tests. Alternatively commercially available algorithms such as FibroTest (BioPredictive, France) are available, although these are less widely used in Australia. There may be some advantage to using a combination of tests, particularly when a single test alone is inconclusive.2
What do the results mean?
FibroScan® results range from 2.5 kPa to 75 kPa. Between 90–95% of healthy people without liver disease will have a liver scarring measurement <7.0 kPa (median is 5.3 kPa).
Validation studies, including comprehensive systematic reviews of studies that have used liver biopsy as the ‘gold standard’ for assessing liver scarring, have indicated the optimal cut-off for the detection of cirrhosis is around 14 kPa.9,10 A patient with chronic hepatitis C and a liver stiffness >14 kPa has approximately a 90% probability of having cirrhosis, while patients with liver stiffness >7 kPa have around an 85% probability of at least significant fibrosis.9,10 However, the sensitivity and specificity of readings >7 kPa for significant fibrosis are only 79% and 78% respectively10 in patients with chronic liver disease, indicating that FibroScan® cannot completely exclude the possibility of significant liver disease even if the liver stiffness is <7 kPa.
The interpretation of the readings may vary to some degree depending on the liver disease aetiology. Therefore, interpretation of the results is best performed in conjunction with other clinical and/or biochemical parameters, and ideally by someone experienced in managing chronic liver disease.
Case study
A man, 55 years of age, attends the clinic for assessment of his hepatitis C. He has heard that there are new treatments available and is keen to find out more. His hepatitis C infection was acquired through a brief period of intravenous drug use over 30 years ago.
His physical examination is unremarkable for the presence of chronic liver disease, and his haematological and biochemical assessments indicate he has hepatitis C genotype-1a.
His ALT = 78 U/L, AST = 81 U/L, albumin = 34g/L, platelet count is 145 x 109/L, and an INR = 1.3. An abdominal ultrasound indicates a diffusely fatty liver measuring 13 cm.
A FibroScan® is performed (Figure 3), at which time 12 valid readings (100% success rate) were obtained from the right lobe of the liver, with a median liver stiffness of 26 kPa.
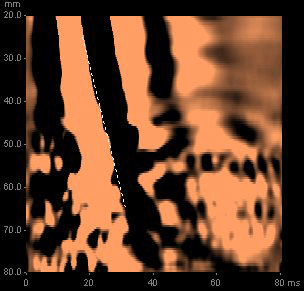
Figure 3. An elastogram demonstrating a liver stiffness of 26 kPa
On the basis of this reading, a low normal platelet count and his history of chronic hepatitis C, he was informed that his probability of cirrhosis was >95%.
He entered a hepatocellular carcinoma screening program of 6 monthly liver ultrasounds and underwent a gastroscopy, where he was found to have small oesophageal varices. He also decided to treat his hepatitis C with the aim of eradicating the virus, which would substantially reduce his risk of future development of hepatocellular carcinoma, liver decompensation and liver related death.
Case comment
This case highlights the important utility of FibroScan® in the routine evaluation of patients with suspected liver disease. The test enabled the diagnosis of liver cirrhosis to be made, which in turn led to the establishment of key surveillance programs for varices and liver cancer. In addition, the detection of advanced liver disease facilitated the decision to undertake antiviral treatment to reduce the risk of developing complications in the longer term.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.