Anaphylaxis is primarily a clinical diagnosis. Recognising anaphylaxis can sometimes be problematic, for example, in the absence of an obvious trigger. In addition, skin and mucosal features can be absent in up to 10–20% of episodes.6 Serial serum tryptase levels can sometimes assist in confirming anaphylaxis in unclear cases.5,6 Anaphylaxis should be considered in any patient acute respiratory distress, bronchospasm, hypotension and/or cardiac arrest (Table 1).5
Table 1. Common features of anaphylaxis
| Respiratory/airway | Cardiovascular | Skin | Gastrointestinal |
|---|
Stridor/wheeze
Difficulty swallowing
Persistent cough
Dyspnoea
Hoarse voice
Throat/chest tightness |
Tachycardia/bradycardia
Collapse/loss of consciousness
Hypotension
Pale and floppy (in infants) |
Urticaria (hives, wheals)
Angioedema
Flushing
Generalised itch |
Nausea
Vomiting
Abdominal pain
Diarrhoea |
Epidemiology
Internationally, lifetime prevalence of anaphylaxis has been estimated to be 0.05–2.0%.7 In Australia, anaphylaxis presentations are increasing (Figure 1).8 However, deaths from anaphylaxis remain relatively rare, with 112 (mostly adult) identified deaths between 1997–2005 (Figure 2).8
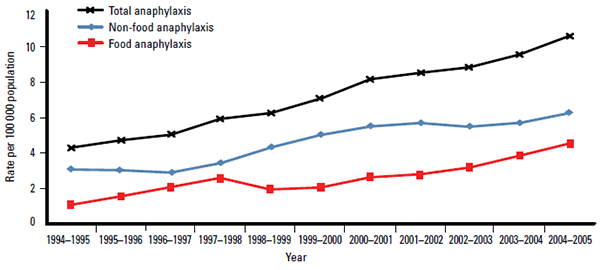
Figure 1. Time trends in anaphylaxis admissions in Australia, 1994–2005
Note: Non-food anaphylaxis includes medication-induced, probable medicationinduced, insect venom-induced, of undetermined cause and relating to a medical or surgical procedure
Adapted from Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol 2009;123:434–42
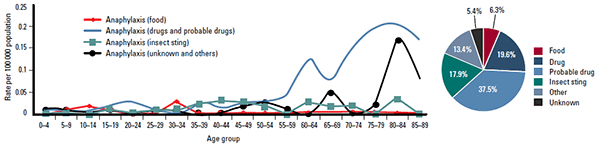
Figure 2. Causes of anaphylaxis fatalities by age group, Australia 1997–2005
Adapted from Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol 2009;123:434–42
Causes of anaphylaxis
Medications, food and insect venom are the most common triggers for anaphylaxis.
Medications are a common trigger for anaphylaxis hospitalisation in older adults (Figure 3), and disproportionately contribute to anaphylaxis deaths in Australia (57%) (Figure 2).8 Medications that most commonly trigger anaphylaxis are antibiotics (especially penicillins), anaesthetic drugs, non-steroidal anti-inflammatory drugs (NSAIDs) and opiates.9
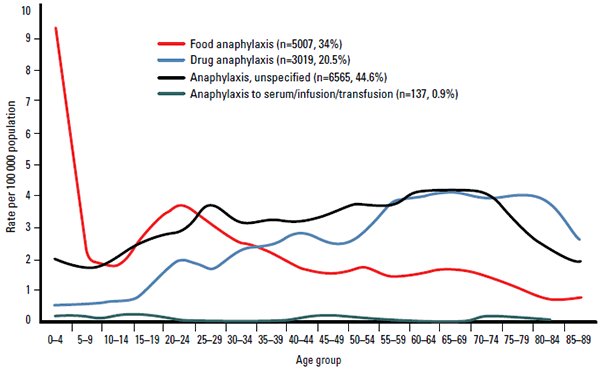
Figure 3. Causes of anaphylaxis admissions by age group, Australia 1994–2005
Adapted from Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol 2009;123:434–42
Food is the most common trigger for anaphylaxis in children.4 Hospitalisation for food-related anaphylaxis is most common in the 0–4 years age group in Australia, with a second peak in the 15–29 years age group (Figure 3).8 Overall, food allergies caused only 6% (n=7) of all anaphylaxis deaths between 1997–2005, six of whom were aged more than 10 years (Figure 2).8 The foods that most commonly trigger anaphylaxis are peanuts, tree nuts, hen’s eggs, cow’s milk, wheat, shellfish, fish and seeds (eg. sesame).10 Nut allergies carry the highest risk of anaphylaxis and death from anaphylaxis.4,10,11
The venom of bees, wasps, and certain types of ants can trigger anaphylaxis. Insect stings were associated with 18% (n=20) of anaphylaxis deaths between 1997–2005, mostly in males aged more than 35 years (Figure 2).8 Australia-wide data regarding insect sting related anaphylaxis presentations and hospital admissions remain limited.8
Note that exercise-induced anaphylaxis can occur in association with a food trigger or in isolation.6 Other less common triggers of anaphylaxis such as latex, radiocontrast media and idiopathic anaphylaxis will not be specifically addressed here.
Risk factors
Risk factors for developing anaphylaxis
Some features in a patient’s medical history may indicate an increased risk of anaphylaxis, including previous anaphylaxis, multiple drug allergies, nut allergy and a history of asthma (especially if poorly controlled).10,12,13
In addition, certain factors present around the time of allergen exposure can increase the risk of anaphylaxis. These include alcohol, exercise, NSAID use and intercurrent infection.5,6,11,14
There are currently no tests that predict anaphylaxis risk.15 While predictive for clinical allergy, the level of allergen specific IgE or size of the skin prick testing (SPT) reaction do not correlate with risk of anaphylaxis.3,6
Risk factors for increased severity or fatality
A number of factors influence the risk of fatal anaphylaxis, including the severity of underlying allergy, allergen dose, patient age, medical comorbidities and concurrent medication use.6,11 Asthma and cardiovascular disease in particular are associated with an increased risk of severe or fatal anaphylaxis.6,10,11,14 The use of concurrent medications, such as beta-blockers and angiotensin converting enzyme inhibitors (ACEIs), can also increase the severity of anaphylaxis and/or render anaphylaxis more refractory to treatment.6,11,15 Risk factors for fatality vary according to the cause of anaphylaxis (Table 2).
Table 2. Risk factors for fatal anaphylaxis by trigger
|
Medication – older adult age group (55–85 years), antibiotic or anaesthetic trigger, cardiovascular and respiratory comorbidities, concurrent medications (eg. ACEI, beta-blockers)6,8
Food – adolescent and young adult age group (10–35 years), active asthma, peanut trigger, ingestion of food not prepared at home6,8,19,20
Insect venom – adult age group (35–85 years), being male (likely to be related to increased risk of exposure)8
|
Management
Acute management
Adrenaline is first line treatment for anaphylaxis.16 Table 3 outlines the emergency management of anaphylaxis. Intramuscular (IM) injection into the anterolateral thigh is the preferred route for the initial administration of adrenaline (Table 4).10
Additional supportive therapy with nebulised beta-2 agonists (for bronchospasm), H1 antihistamines (for cutaneous symptoms), and/or glucocorticoids (may reduce the risk of biphasic reactions) is often utilised in clinical practice, but plays a less important role and is considered second line.6,10,17 These medications should never be used as an alternative to, or before, adrenaline for anaphylaxis.15
Table 3. Emergency management of anaphylaxis
| 1. Stop exposure to causative agent (if possible), assess reaction severity and treat accordingly |
|---|
- Call for assistance
- Give adrenaline IM (lateral thigh) 0.01 mg/kg (maximum adult dose 0.5 mg)
- Lay patient flat (elevate legs if tolerated)
- Set up IV access
- Give high flow oxygen + airway/ventilation support if needed
- If hypotensive, also:
- set up additional wide bore IV access (14 G or 16 G in adults) for normal saline infusion
- give IV normal saline bolus 20 mL/kg stat
|
|
2. If there is inadequate response, an immediate life threatening situation or deterioration:
|
|---|
- Repeat IM adrenaline injection every 3–5 minutes, as needed, or
- Start an IV adrenaline infusion, as per hospital guidelines/protocol*
|
|
* IV adrenaline infusion usually requires intensive care expertise for administration. Intravenous bolus administration of adrenaline is best avoided
Adapted from Brown SG, Mullins RJ, Gold MS. Anaphylaxis: diagnosis and management. Med J Aust 2006;185:283–9
|
Table 4. Benefits of IM injection as route for initial dose of adrenaline3,21
- Rapid and effective absorption
- Avoids time delay in getting IV access
- Less monitoring needed
- Less potential for significant adverse effects
For adrenaline autoinjectors specifically:
- Able to be safely administered by non-health professionals (with appropriate training)
- Lower risk of dosing errors
|
Long-term management
General practitioners play a central role in the long-term management of patients with anaphylaxis. Long-term management includes the following steps.1
Referral to an allergy specialist
All patients with anaphylaxis should be referred to an allergy specialist (allergist/clinical immunologist) for further assessment and evaluation.1
Identification of trigger(s)
Accurate identification of the causative allergen guides appropriate management and enables future avoidance. History taking should cover recent medication use (including complementary medicines), food intake and/or insect stings, as well as any relevant co-factors.
Following this, SPT and/or blood allergen specific IgE (sIgE) testing to the suspected allergen may be indicated.18 Tests should be interpreted using standardised criteria. For SPT, appropriate safety precautions should be in place. Screening SPT and sIgE testing (in the absence of a history of allergic reaction and identification of an implicated allergen) is discouraged.
Where no triggers are identified on history together with negative SPT or blood sIgE testing, a diagnosis of idiopathic anaphylaxis may be considered, but this is less common.6
Avoidance of trigger(s)
Avoidance of allergens is essential to minimise risk. Allergen-specific strategies are outlined below.
Medications
- Document drug allergies clearly in the patient’s file
- Provide wristband alerts if the patient is hospitalised
- Suggest the patient wears a MedicAlert bracelet
- Inform the patient when prescribing medications that have a particular risk of anaphylaxis, consider an observation period after first dose.
Food
- When eating food prepared outside the home, ask about the ingredients used
- Read ingredient labels carefully
- Be aware of situations that carry a high risk of cross-contamination, eg. buffet service, use of shared utensils (eg. ice-cream, milkshake, juice bars, deep fryers)
- For adolescents and adults, consider ‘cautious touch-testing’ where food is initially touched to the lips – any development of tingling, rash or swelling may indicate allergy12
- Consider dietician input to assist with dietary management.
Insect stings
- Wear closed shoes, long pants and long-sleeved shirts when outdoors
- Wear gloves when gardening
- Avoid provoking bees or wasps where possible.
Prescription of adrenaline autoinjector
Patients should be assessed for the need for an adrenaline autoinjector (EpiPen® or Anapen®). All patients who have experienced an anaphylactic reaction and have continuing risk of exposure to an allergen trigger should be prescribed an adrenaline autoinjector. For example, those with food or insect related anaphylaxis usually require an adrenaline autoinjector, whereas those with medication related anaphylaxis are not commonly prescribed an adrenaline autoinjector as the trigger can be more easily avoided. Prescribing guidelines can be found at www.allergy.org.au (see Resources). Provision of an adrenaline autoinjector must be accompanied by education for patients and carers about when and how to use the device, and provision of an anaphylaxis action plan (see next step).
The Pharmaceutical Benefits Scheme allows for Authority prescriptions of adrenaline autoinjectors (maximum quantity of two with no repeats). Approval is provided for individuals assessed as being at significant risk of anaphylaxis (with the name of the consulting clinical immunologist, allergist, paediatrician or respiratory physician) and patients who have received adrenaline for treatment of anaphylaxis.1
Written emergency action plan for anaphylaxis
All patients who have had anaphylaxis and remain at risk of further allergic reactions should have a personalised emergency action plan that outlines the emergency management of allergic reactions. Patients who have been prescribed an adrenaline autoinjector should have a personalised emergency action plan for anaphylaxis (see Resources). This plan should be specific to the prescribed adrenaline autoinjector (Epipen® or Anapen®). (Note: patients who have experienced a mild to moderate allergic reaction but not anaphylaxis and who do not have an adrenaline autoinjector should also be provided with an emergency action plan for allergic reactions
The written emergency action plan for anaphylaxis should include:
- patient and emergency contact details
- a list of specific allergens for the individual patient
- doctor’s contact details and signature
- date the plan was prepared (to be updated annually)
- information on features of anaphylaxis and appropriate emergency management.
- Patients should receive accompanying verbal education regarding symptom recognition and emergency management, using the written plan as a reference. For children, a copy of the plan should be given to family members, teachers and other carers.
Regular follow up
Annual review with a regular family doctor is an important aspect of ongoing care. At the annual visit, the GP should:
- monitor for repeat allergic reactions
- reinforce behaviours to avoid triggers
- review and optimise management of comorbidities, particularly asthma and cardiovascular disease10,12
- review medications that may complicate management of allergic reactions
- consider the need for further allergen testing (eg. to monitor for resolution of food allergy)
- re-educate the patient and their family to ensure they can correctly recognise and manage allergic reactions
- re-educate the patient and their family on the use of the adrenaline autoinjector if one has been prescribed OR review the need for adrenaline autoinjector prescription if one has not been prescribed
- update the personalised emergency action plan for anaphylaxis.
Repeat follow up with an allergy specialist may be considered if new symptoms develop, confirmation of allergy resolution is required, allergies are difficult to manage and/or during periods of increased risk. Relevant periods of increased risk may include adolescence, leaving home or travel, and changes in health status and comorbidities. The specialist may suggest additional tests or management strategies as appropriate.
Key points
- Presentations and hospital admissions for anaphylaxis are increasing, however, death from anaphylaxis is rare.
- The most common causes of anaphylaxis are medications, food and insect venom. Medications are the most common cause of anaphylaxis in older adults, particularly antibiotics, anaesthetic drugs, NSAIDs and opiates. Food allergy is the most common cause of anaphylaxis in children, but rarely results in death.
- Anaphylaxis is a medical emergency requiring immediate treatment with adrenaline.
- Long term management is important to minimise ongoing risk. This includes referral to an allergy specialist, identification of the trigger, allergen avoidance, consideration of an adrenaline autoinjector, provision of an emergency action plan with accompanying education, and regular annual follow up.
- General practitioners have a key role to play in the identification, management and prevention of anaphylaxis.
Resources
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.