There are two primary goals of recruitment: recruitment of a sample that is representative of the target population, and recruitment of a sufficient number of participants to meet both sample size and power requirements.7 However, budgetary, staffing and time constraints are all challenges to the logistics of conducting a study.3 Pilot studies, although costly and time consuming, play an important role in identifying any difficulties before commencement of large studies.
It is recognised that people who decline to participate in studies tend to have different characteristics from those who volunteer their participation.3 Poor recruitment rates are commonly associated with factors such as increased age, male gender, non-Caucasian background, low education, low income or unemployment, smoking status, recent illness or poor health.8,9 Older adults are under-represented in almost all health related research, as both ethical and logistical challenges exist in this cohort.10–13 Older participants may have multiple comorbidities with frequent medical appointments; suffer from fatigue as a result of participation; have cognitive impairment that makes obtaining consent, recruitment and involvement more complex; or are homebound and have difficulties with travel to the study site.14 In Australia, older people represent a growing proportion of society.15 Nevertheless, little attention has been given to the sampling and recruitment challenges that researchers encounter when trying to enlist participation of older community-based adults.
In this context, we aimed to profile non-respondents of a case control study investigating warfarin safety in community-based patients, and to outline the lessons learnt from strategies implemented to improve recruitment after analysing challenges faced in a previous pilot study.
Methods
Study design and population
This is a subanalysis of a case control study designed to investigate the risk factors associated with bleeding risk in patients prescribed warfarin. The study methods and results of this warfarin study have been published previously.16
The study population comprised community-based patients aged ≥18 years who were stabilised on warfarin for a minimum of 3 months. All patients were managed by a large metropolitan pathology provider based in Melbourne, Victoria, and were approached during routine warfarin monitoring appointments. Data was collected on participants and non-participants for this substudy, comprising age, gender and reasons for non-participation.
Four attempts were made to contact eligible patients at different times of the day, including early evening. Patients were called on the contact number provided by the pathology provider. (This number is updated frequently to minimise adverse events in cases of warfarin instability that requires dosage adjustment.) All patients were recruited within 30 days of the initial approach.
Recruitment strategies
The recruitment pathway is outlined in Figure 1. A previous pilot study identified areas in the recruitment process that were potentially challenging. As a result, a number of strategies were implemented in the case control study to improve patient recruitment and also to facilitate stakeholder involvement.
Announcements placed in the newsletters of divisions of general practice raised stakeholder awareness of the project.
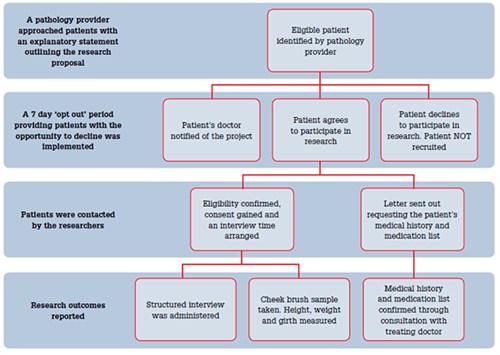
Figure 1. Recruitment pathway used for pathology provider-dosed patients
Identifying patients for the study
Patients were identified by the pathology provider during routine monitoring appointments. An additional staff member was employed for this role. A personalised letter from the haematologist involved in the patient’s care was sent from the pathology provider, which was followed up with a telephone call that allowed for explanation of the nature of the research and the requirements of participation. A follow up explanatory statement was then mailed to potential participants.
GP involvement in the study
The pathology provider notified the patient’s general practitioner, by telephone and with a detailed letter outlining the research study, when the patient was initially contacted. The GPs were encouraged to discuss the project with their patient, the pathology provider or the researchers. The GPs could also recommend that their patients not be involved in the research if they felt participation was inappropriate. General practitioners were directly involved in the study by way of providing additional information about their patient’s medical history and medications. The recruitment rate for this information from GPs was 91% (443/486 GPs).
‘Opt out’ method
An ‘opt out’ method was used whereby patients were given a 7 day period after the initial invitation to decline to participate, by either calling the pathology provider or by mailing a card. ‘Opting out’ prevented any further contact. Ethically, it is important to distinguish that the ‘opt out’ method was used only in the initial approach and not in the consent process. After the 7 days, if the patient had not ‘opted out’ they then received further contact and detailed information to assist them in their decision-making process.
Interview attendance
Patients were given the choice of a home visit for the interview or attendance at a local site. Taxi vouchers and parking reimbursements were available to assist with transport issues.
Reasons for non-participation in this study were analysed. Data were analysed according to age, gender, and reason for declining or inability to participate.
Results
Of 734 eligible patients, 486 participants were recruited between 1 March 2008 and 30 July 2009 (recruitment rate 66%), with 247 participants declining involvement. The median age was 80 years (range 43–98 years); 47% were female. Table 1 compares the age and gender distribution of the recruited participants and the non-recruited participants. Patients who were recruited to the study did not markedly differ from those who declined; and there was little difference between recruited and non-recruited cases and controls in relation to all variables collected on unenrolled patients, ie. age (median and range), gender, International Normalised Ratio level and warfarin dose.16 There were older patients that were not recruited.
Reasons for non-participation were:
- 115 (47%) used the ‘opt out’ method
- 57 (23%) stated they were too unwell
- 39 (16%) were not recruited due to a health professional’s recommendation
- 36 (14%) were unable to be contacted.
Table 1. Comparison of recruited and not recruited participants by age and gender
| Age (years) | Non-recruited participants
n=247 (%) | Non-recruited
(33.7% of all patients approached) | Recruited participants
n=486 (%) | Recruited
(66.3% of all patients approached) |
|---|
Male
(53%) | Female
(47%) | Total | Male
(57%) | Female (43%) | Total |
|---|
| ≤65 |
9 (7) |
7 (6) |
16 (6.5%) |
43 (15) |
23 (11) |
69 (14%) |
| 66–79 |
44 (33) |
30 (26) |
74 (30%) |
135 (49) |
99 (47) |
234 (48%) |
| ≥80 |
79 (60) |
78 (68) |
157 (63.5%) |
99 (36) |
87 (42) |
186 (38%) |
| Total |
132 (100) |
115 (100) |
|
277 (100) |
209 (100) |
|
Discussion
The distribution of reasons for not participating in the study were similar to those noted in other community-based research, with illness and ‘opting out’ being prominent reasons.9 Our results show that patients aged ≥80 years were less likely to participate, with almost two-thirds of our non-responders being in this age group.
Recruitment strategies, such as those outlined in Table 2, including an ‘opt out’ approach and recruitment through a third party provider, enabled us to successfully recruit a generalisable sample.
Table 2. Suggestions for recruitment strategies in non-interventional research
- Provision of an informative outline of the patient and doctor’s required commitments to the research study
- Use of a third party/proxy to assist with the research involvement
- Use of an ‘opt out’ approach for patients who do not wish to receive information. This allows for focus on patients interested in participation
- Regular communication with stakeholders (eg. GPs and specialists) about research progress
- Personalised communication with patients and doctors from a relevant, known, and trusted stakeholder who has an independent relationship with the patient before the research
|
Successful recruitment is dependent on many factors. One element we relied on was an understanding that this research would improve treatment for patients prescribed warfarin in the future. Warfarin is a complex anticoagulant that requires regular blood tests and at times, dosage adjustment to keep anticoagulation levels within the warfarin therapeutic window.17 Therefore in the warfarin population, the pathology provider has a long-standing and regular relationship with patients and their GPs.
In our study, the pathology provider contacted the GP to invite them to collaborate. This allowed researchers the unique opportunity for contact by a well-known third party. This relationship was the foundation of the successful recruitment of patients in a timely fashion. By partnering with a third party and collaborating with GPs, patients were given unbiased support and information regarding the research study. This partnership further allowed GPs to be involved in the research, but also minimised any administrative burden, which was the responsibility of the researcher and pathology provider.
An important part of the recruitment process encouraged GPs and patients to discuss their potential involvement in the research. In all cases where health professionals discouraged patient participation, the GP had discussed the research with the patient. Reasons cited included concern that the research would be too burdensome for the patient and therefore compromise the patient’s health, or the patient’s ill health. This is in keeping with other community-based research and is particularly problematic when recruiting in an ageing population where comorbidities are prevalent.5
The use of an ‘opt out’ method is appropriate and ethical, allowing the research to remain feasible. By approaching patients who are actually interested in being involved, recruitment is optimised. Allowing patients to ‘opt out’ and using a third party to recruit patients are both innovative successful tools that we used in our research. In the previous pilot study we attempted recruitment initiated directly by a telephone call from the pathology provider. This proved to be costly and inefficient. The ‘opt out’ approach improved our recruitment rate while not being a substitute for written consent. Participants could withdraw at any point, even after the interview had been completed, and have their details removed. In non-interventional research, an ‘opt out’ allows for targeted recruitment and better time management by researchers.
Before recruitment, potential participants and their doctors were informed what their role would be, particularly the time and travel commitment and possible benefits of involvement. This was achieved through direct communication and collaboration using correspondence and follow up telephone calls.
Patel et al18 describe these complexities and emphasise the importance of understanding the patient’s perspective, for whom research may be perceived as an unfamiliar and demanding experience.4 Lengthy questionnaires, frequent appointments with inconvenient costs, time and travel can make patients reluctant to participate. Accommodating and understanding the patient’s circumstances is necessary. By partnering with a pathology provider, participants could attend a familiar location during regular warfarin visits. Alternatively, they could select home-based interviews. Participants were reimbursed for their parking costs, or for travel via taxi vouchers.
Limitations of this substudy include the limited data available on non-participants. In particular, information regarding patients who were uninterested in the research was limited, and it would be of value to gain more information about the non-recruited group to enhance our understanding of their reasons for non-participation. Further, the inability to include seriously unwell or non-contactable patients could have created a selection bias in our study. These issues have important implications for generalisability of the findings. However, the ability to recruit these patients is not within the researchers’ control. Nevertheless, our recruitment rate improved markedly from our pilot study and provided the main study with a representative sample.
Conclusion
With an ageing population, steps need to be considered to ensure older patients are represented in community-based non-interventional research. During development of the study design, investigators need to be aware of potential barriers that may hinder the recruitment of patients and be particularly aware of older participants. Provisions must be made for additional resources such as taxi vouchers and convenient study sites for participants.
The lessons learnt from this research show that multidimensional partnerships that go beyond researcher and doctor and include a familiar third party, could be of benefit to all stakeholders. In addition, an ‘opt out’ approach when not used as a substitute for consent, makes recruitment more feasible and decreases the administrative burden for doctors. Sharing of the recruitment responsibilities also allows for strengthening of existing professional relationships.
Key points
- Recruitment is one of the most challenging aspects of non-interventional research.
- For a full evidence base, elderly patients need to be represented in research outcomes.
- Using a known, trusted third party, such as a pathology provider or GP, can result in successful and feasible recruitment.
- A multidimensional partnership between GPs, researchers and pathology providers could balance the difficulties faced by non-interventional research projects.
Competing interests: None.
Ethics approval: Human Research Ethics Committees of Monash University and Cabrini Health.
Funding: This work was supported by a National Health and Medical Research Council grant (436763).
Provenance and peer review: Not commissioned; externally peer reviewed.
Acknowledgements
We would like to acknowledge Melbourne Pathology for assistance with recruitment, and the patients and GPs who participated in the research.