The school-based catch-up was completed at the end of 2008. The community-based catch-up ceased on 30 June 2009, with women able to access vaccine for completion of the course until 31 December 2009. As of 2013, the program is school-based, targeting girls and boys aged 12–13 years, with a two-year catch-up program for boys aged 14–15 years currently underway.4 Although the program is now school-based, there are many reasons why general practitioners (GPs) may still deliver HPV vaccinations, such as school absence, catch-up or family preference.
The National HPV Vaccination Program Register (NHVPR) began operating in June 2008. The NHVPR collects HPV vaccine notifications across Australia for all ages and sexes. Notification is strongly encouraged but not mandatory for GPs. The NHVPR helps ensure vaccine courses are completed by providing overdue dose reports to providers, and history statement reminders and completion statements to consumers. The NHVPR is also vital for evaluating the program, monitoring vaccination coverage and, ultimately, measuring the impact on HPV-related disease.1 It will also provide a recall system if booster doses of vaccine are ever required.
GPs are able to notify HPV doses directly to the register in multiple ways (Table 1). Queensland (Qld) and the Northern Territory (NT) are exceptions, having state-based immunisation registers that GPs report to and that send notifications on to the NHVPR.5 During the catch-up program, GPs registered with the NHVPR received $6 per notified dose.6 These payments ceased on July 2010.
This paper presents HPV vaccine coverage rates by DGPs for the first time. A number of sources point to under-notification of HPV vaccination in adult women during the catch-up, with data suggesting around 10% under-notification across Australia.5,7 For this reason, NHVPR coverage data by DGPs are presented here alongside a survey exploring under-notification to better interpret the coverage rates.
Table 1. Notifying HPV vaccine doses
| Methods of reporting |
|---|
| 1. Online portal
|
2. Document-based: faxed or posted (disc or printout) to the register
Single dose
- Vaccination notification form (single dose)
Multiple doses
- Vaccination notification form template (multiple doses)
- Reports generated from practice software
|
| Requirements for acceptance of notification |
|---|
| 1. Consumer consent (written or verbal) |
2. Minimum fields completed:
- Patient – name, date of birth, address, sex, Medicare number
- Vaccination – date, dose no., vaccine type (Gardasil® or Cervarix®)
- Practitioner – address, provider number
|
3. Other non-mandatory fields requested:
- Patient – Indigenous status
- Vaccination – batch number
|
Note that entering HPV vaccination details into practice software does not automatically result in notification to the NHVPR.
Consumers can opt-off being on the Register at any time.
The online portal, forms and templates are all accessible at hpvregister.org.au |
Methods
DGP vaccine coverage rates
All notified HPV vaccine doses administered to women aged 18–26 years in 2007 (ie. eligible for the catch-up program) were extracted from the NHVPR as at 29 November 2011 as de-identified data at the postcode level, based on consumer address. Data were converted to 2008 DGP boundaries. Vaccine coverage was calculated by dividing notified doses by the Australian Bureau of Statistics 2007 Estimated Resident Population of women aged 18–26 years for each DGP area. Spatial analyses used ESRI ArcMap version 10 software.
Immunisation coordinator survey
A pilot online survey was tested with three immunisation coordinators. Once refined, a unique link to the online survey was emailed to 118 DGP immunisation coordinators, representing all 112 Australian DGPs. The survey consisted of 20 questions relating to division characteristics and immunisation practice, the percentage of practices believed to notify HPV vaccine doses, common features of non-notifying practices and barriers to notification, with the opportunity to comment on the NHVPR. The initial email was sent on 7 September 2010, with a reminder to non-responders 1 month later. The survey was promoted via weekly fax for 3 weeks before, during and after the initial email. Of the four divisions where more than one contact was emailed, only one coordinator responded per DGP.
Additional demographic information about participating DGPs was obtained from the 2010–2011 Annual Survey of Divisions (ASD).8 To obtain the proportion of GPs registered with the NHVPR, the number of GPs registered as of 28 January 2011 was divided by the number of employed GPs for each DGP per ASD data.
Survey responses were analysed using STATA v10. Group comparisons were made using two-sample tests of proportion (for proportions) and two-sample t-tests with equal variance (for means). Missing data was excluded from all statistical comparisons. Replies to open-ended questions were coded and analysed by theme.
Ethics approval was not required for this study as it was a quality assurance project. The study was approved by the Commonwealth Department of Health and Ageing, the owners of the NHVPR.
Results
HPV vaccine coverage rates across DGPs are shown in Figure 1a–c, with DGPs divided into quintiles. The range across DGPs nationally was 22–70% for dose 1, 13–60% for dose 2 and 7–49% for dose 3.
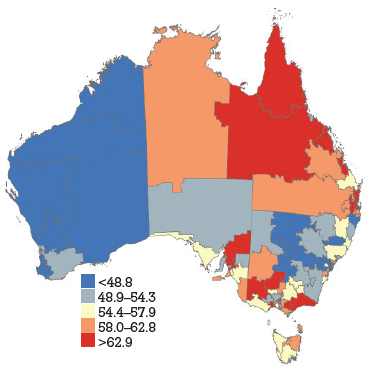
Figure 1a. HPV vaccine uptake dose 1, women aged 18–26 years by Divisions of General Practice, Australia
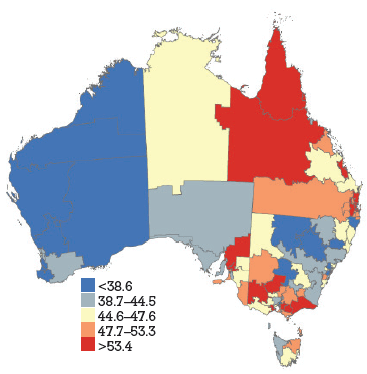
Figure 1b. HPV vaccine uptake dose 2, women aged 18–26 years by Divisions of General Practice, Australia
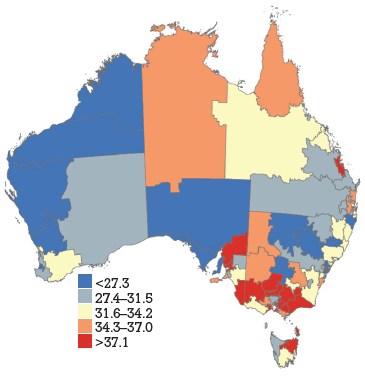
Figure 1c. HPV vaccine uptake dose 3, women aged 18–26 years by Divisions of General Practice, Australia
Immunisation coordinator survey
Characteristics of participating divisions
Of the 112 DGPs in Australia, 58 (52%) participated. Six responders who provided only demographic information were excluded. The state distribution and practice characteristics of participating DGPs were not statistically significantly different from DGPs across Australia (based on ASD data). For example, the mean number of practices was 64.7 (SD 40.5) vs 63.4 (SD 48.2) for Australia (P = 0.86); the mean number of GPs employed was 230.1 (SD 155.0) vs 222.7 (SD 168.5; P = 0.78); the mean GP-to-population ratio was 943.8 (SD 201) vs 956.8 (SD 202.8; P = 0.69). The mean proportion of GPs per DGP registered with the NHVPR was 95.1% (SD 22.0) compared with 95.3% (SD 26.7) for Australia overall (P = 0.96).
Most of the DGPs (n = 52, 90%) reported that other immunisation providers were present within their division, such as family planning clinics (28%), Aboriginal medical services (71%), sexual health clinics (40%) and university medical services (31%).
Vaccination and notification practices
Most practices provided immunisation services, with 42 coordinators (72%) reporting ≥90% of practices provided immunisation and, of those coordinators, 25 (43%) reporting that all practices provided immunisations. Only one quarter (24%, n = 14) of participants were aware of specific practices that did not give any HPV vaccine.
Most participants (n = 38, 66%) believed over 80% of practices in their DGP notified HPV vaccine doses, with 25 (43%) believing >90% notified HPV vaccine doses (Figure 2). Only two divisions reported that <50% of practices notified HPV vaccine doses, 4 (7%) were ‘not sure’, 4 (7%) did not answer. The response pattern by state is shown in Figure 3.
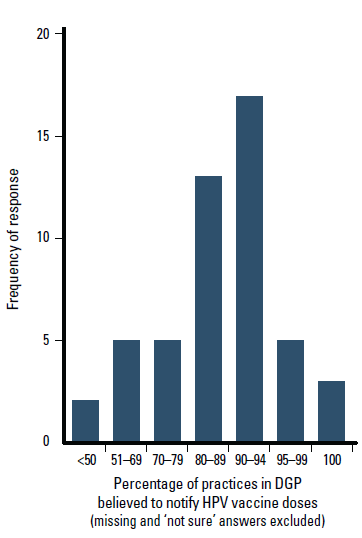
Figure 2. Percentage of practices in DGP believed to notify HPV vaccine doses, as reported by immunisation coordinators
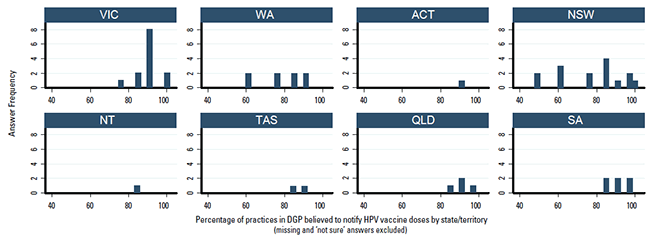
Figure 3. Percentage of practices in DGP believed to notify HPV vaccine doses by state/territory, as reported by immunisation coordinators
There was a positive correlation between survey-reported notification rates and vaccine coverage rates for DGPs (simple linear regression: dose 1, 0.196, P = 0.01; dose 2, 0.199, P = 0.01; dose 3, 0.145, P = 0.02).
Table 2. Ranking: reasons for GPs or practices not notifying HPV vaccine doses
| Ranking | Reason |
|---|
| 1. |
Too busy/did not get around to it |
| 2. |
Unaware of how to notify |
| 3. |
Concerned about patient consent* |
| 4. |
Decided not worth the effort for $6 per dose notification |
| 5. |
Do not provide HPV vaccine |
| *This refers to the situation where patient consent for register notification was not obtained at the time of immunisation. |
Reasons for not notifying HPV vaccine doses
The highest ranked reason why some GPs/practices did not notify HPV vaccine doses was that they were too busy, with 22 (45%) ranking this as the most important reason. The relative ranking of the five-answer options is shown in Table 2. Seven respondents commented that the register not being operational at the start of the program was a significant barrier. Other reasons included not having a practice nurse, being a solo GP practice, difficulties with the register web portal, lack of computer literacy, high staff turnover, deficiencies in infrastructure and staffing, and management processes (Table 3).
Table 3. Immunisation coordinators: comments regarding reasons for not notifying HPV vaccine doses
‘Practices vary (quite significantly) in levels of infrastructure support. Some practices have one receptionist and others have five nurses and eight admin support staff. Practices in this region experience a high turnover of practice staff and nurses, which does not enable continuity of care and business management processes.’
‘I think that the register wasn’t available from the beginning may have been a big issue. It may have improved the process if the register was up and running prior to the commencement of administering the HPV.’
‘Some solo GPs not interested in going through the process of registering for the occasional vaccination. Some multi GP practices may have not registered their locums or registrars after the initial registration process’
‘Time commitment for solo GPs to complete documentation is an ongoing problem’
‘Some solos are better organised than multi practices. Each practice varies in relation to its processes. Some practices have data management processes in place and others don’t.’
|
Discussion
HPV vaccination coverage rates, derived from notifications to the NHVPR, varied widely by DGP but demonstrated some consistent state-wide trends. In general, coverage was higher in Qld, NT and Vic (red and orange), and lower in WA and NSW (blue and grey). It has previously been suggested that differences in jurisdiction notification systems may contribute to apparent differences in coverage between states (with Qld and NT maintaining centralised notification systems).5 The trends across DGPs would also support this hypothesis. Our findings suggest most practices gave HPV vaccine during the catch-up period. The overall ranking of reasons for not notifying emphasises the importance of process barriers (being too busy), with informational barriers (unaware of how to notify) and motivational barriers (not worth the effort) being secondary to this. The delay in NHVPR operation represents a process barrier and may have contributed to concerns about consent.
There was some heterogeneity in coverage within states. In WA, SA, Qld and NSW coverage rates seem higher in metropolitan DGPs within or close to the capital city. In Vic, some DGPs in urban areas have lower coverage rates than rural DGPs. Tas and SA show particular heterogeneity across DGPs. This suggests that notification is influenced by additional factors apart from jurisdictional notification systems (be they practice characteristics or otherwise). The findings also highlight that states with high reported coverage rates overall may still contain DGPs with significantly lower reported coverage rates. For example, SA has one of the highest overall state coverage rates but shows heterogeneity amongst DGPs. Additionally, under-notification by DGP area may also be attributable to incomplete notification from non-GP providers, who did not receive incentive payments for notification. This was not explored in this survey. Register data show that non-GP providers gave 4% of notified doses in women aged 19–26 years during the catch up program. Another caveat in interpretation of DGP rates is that, in this analysis, vaccine doses were allocated on the basis of consumer address rather than practitioner location. This is consistent with population denominator data, which is also based on place of residence. However, place of residence and vaccinating practitioner location may not always be within the same DGP.
Although the response rate was not ideal, we are confident that the results are broadly representative, given the sample’s consistency with ASD data and the inclusion of DGPs with diverse characteristics. Immunisation coordinators’ answers were based on their own experiences of working with practices in their DGP and therefore the basis for answers, particularly regarding the proportion of practices notifying HPV vaccine doses, varied. Few participants (n = 15) were aware of which specific practices did not notify HPV vaccine doses. It is therefore important to acknowledge that there was no uniform, systematic way that exact proportions of practices notifying HPV vaccine doses were reported to immunisation coordinators. This is a limitation of the survey. There may also have been potential for recall bias, given the survey was conducted in 2010. A further consideration is that some practices may have notified some doses but not all: this issue was not explored. Finally, despite the usefulness of immunisation coordinator responses, they are an indirect assessment of under-notification. It would have been ideal to randomly survey GPs directly. Due to a number of restrictions, this broader study was not possible. Nevertheless, there was a positive correlation between survey-reported notification rates and vaccine coverage rates for DGPs, supporting the validity of immunisation coordinator estimates. The consistency is also borne out in overall state estimates of notification (survey reported) and vaccine coverage (notified to NHVPR), with NSW and WA ranked lowest for both measures. However, the exact magnitude of under-notification cannot be inferred from this survey. Other research is required and should include population-based surveys7 and validation studies of self-reported HPV vaccine receipt compared with register records.9 Our findings do, however, support the assertion that under-notification was a significant issue in general practice during the catch-up period.
The results underline the value of information and guidance for healthcare providers about notification. Even more so, they emphasise the importance of timely, adequate and efficient processes to support notification. The importance of process extends from individual practice management, divisional support and state-based notification mechanisms through to the operation of the NHVPR. Some practice characteristics may be more common amongst notifying practices. However, as responses indicate, practices vary greatly in their staffing and organisation. Motivation and having an articulated process for notification may be more important than any particular mechanism or clinic resource.
Conclusion
DGP coverage rates demonstrate consistent state-wide trends, as well as heterogeneity within states. The survey findings provide a useful context for interpreting DGP vaccine coverage rates during the HPV catch-up program and a better understanding of barriers to notification in general practice. These findings should be useful in evaluating the HPV catch-up program, improving notification in the current HPV vaccine program and informing the implementation of other vaccine registers and adult vaccination programs in the future.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Acknowledgements
Many thanks to the participating immunisation coordinators Helen Moore, Hailey Shaw, Greg Rowles, Silvia Hope, Lesley Rowlands and Penelope Allen for their assistance with the survey.