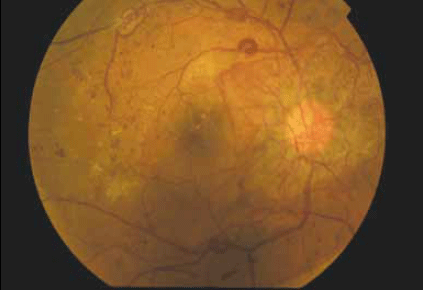
Figure 1. Right fundus photo
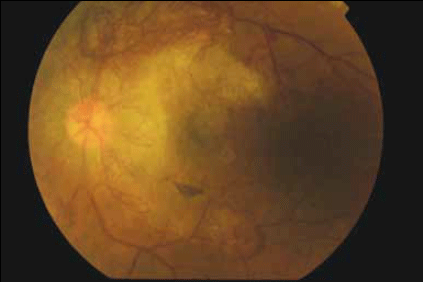
Figure 2. Left fundus photo
Question 1
Describe the fundus findings.
Question 2
What dilating drops would you use to perform a dilated fundus examination? What precautions would you consider when using dilating drops?
Question 3
What systemic disease is most likely responsible for the fundus findings?
Question 4
What is the mechanism of visual impairment in this condition?
Question 5
What ophthalmic treatment options are available for this patient?
Question 6
What parameters would you monitor and manage in this patient to reduce his ongoing risk of complications?
Answer 1
The fundus photos demonstrate severe proliferative diabetic retinopathy (DR) in both eyes. The diagnosis of proliferative DR is based on the presence of abnormal blood vessels that grow in response to elevated levels of vascular endothelial growth factor (VEGF) from retinal ischaemia. These vessels can occur either at the optic disc (known as NVD – new vessels at the disc) or elsewhere on the retina (known as NVE – new vessels elsewhere). This patient has gross neovascularisation in both eyes, ie. predominantly concentrated on the optic disc bilaterally. In the left eye, new vessels are also present along the superior and inferior temporal vascular arcades.
In addition to the proliferative disease, other features of DR are seen: there are numerous microaneurysms and intraretinal hemorrhages in both eyes; a striking omega loop along the right superotemporal vascular arcade and a second along the right inferotemporal arcade, indicating vascular dysfunction; and two vascular abnormalities evident in the right superior temporal quadrant. These may be intraretinal microvascular abnormalities (IRMA) or NVE. An IRMA is a sign of severe nonproliferative DR due to vessel blockage and may often precede neovascularisation.
Temporal to the right fovea, an old thrombosed vessel can be seen. Diabetic macular oedema is present in both eyes indicating vascular leakage. There are hard exudates near the right fovea, seen as yellow intraretinal deposits. 'Cottonwool spots' may be another feature of DR and represent areas of retinal ischaemia. However, these are not present in this case.
There are several grading systems available to assess the severity of DR. A clinically useful system for general practitioners is the International Clinical Diabetic Retinopathy and Diabetic Macular Oedema Disease Severity Scale,1 developed by the American Academy of Ophthalmology. A summary of this classification is shown in Table 1. It is important to note that the presence of macular oedema (and associated vision loss) is independent of the severity of the DR. Indeed, vision loss from macular oedema can occur even in mild or moderate nonproliferative DR.
Table 1. International Clinical Diabetic Retinopathy and Diabetic Macular Oedema Disease Severity Scale1
| Retinopathy stage | Findings on fundoscopy |
|---|
| No apparent DR |
No abnormalities |
| Mild nonproliferative DR |
Micro-aneurysms only |
| Moderate nonproliferative DR |
More than micro-aneurysms only, but less than severe nonproliferative DR |
| Severe nonproliferative DR |
Any of the following:
- more than 20 intraretinal haemorrhages in each of four quadrants
- definite venous beading in 2 or more quadrants
- prominent intraretinal microvascular abnormalities in 1 or more quadrants and no neovascularisation
|
| Proliferative DR |
One of the following:
- neovascularisation
- vitreous or preretinal haemorrhage
|
| Macular oedema | Findings on fundoscopy |
|---|
| Absent |
No retinal thickening or hard exudates at the posterior pole |
| Present |
Mild – some retinal thickening or hard exudates in posterior pole but distant from the centre of the macula
Moderate – retinal thickening or hard exudates approaching but not involving the centre of the macula
Severe – retinal thickening or hard exudates involving the centre of the macula |
Answer 2
A suitable dilating drop for an office based adult dilated examination is one drop of tropicamide 1% in each eye. The time to maximal effect is 20–30 minutes2 so the drops may be repeated after 20 minutes if the appropriate dilation is not achieved. Tropicamide may be used alone or in conjunction with 2.5% phenylephrine, one drop in each eye. In patients with poorly controlled hypertension, phenylephrine should be used with caution as there may be systemic side effects.3 A summary of the available dilating drops is shown in Table 2.
Table 2. Dilating eye drops, with approximate time for maximal effect and duration of action2
| Medicine and available strengths | Approximate maximal effect | Approximate duration of action |
|---|
| Phenylephrine hydrochloride 2.5%, 10% |
20 minutes |
3 hours |
| Tropicamide 0.5%, 1% |
20–30 minutes |
3–6 hours |
| Cyclopentolate 0.5%, 1% |
20–45 minutes |
24 hours |
| Homatropine 2% |
20–90 minutes |
2–3 days |
| Atropine 0.5%, 1% |
30–40 minutes |
1–2 weeks |
Before pharmacological pupil dilation, it is important to check the pupillary reflexes to light and in particular to exclude a relative afferent pupillary defect (using the so-called 'swinging torch test'), as this can be an important indicator of retinal or optic nerve pathology. Importantly, the risk of inducing an attack of acute angle closure by pharmacological dilation is estimated to be less than 1:20 000.4
There is a small risk of systemic absorption of all eye drops and care should be taken in pregnant and breastfeeding women. If necessary, the 'double DOT technique' (combination of 'don't open the eyelid' and 'digital occlusion of the tear duct') may reduce systemic absorption.5
Patients need to be warned that dilated examination may cause blurring of vision and difficulty reading until the effects of the drops wear off, and they should not drive during this time.
General practitioners should be aware that the direct ophthalmoscope gives a highly magnified view of the retina with a small field of view, and that it may be difficult to visualise the peripheral retina.6 Regular practise is needed for a skilled examination.
Answer 3
Diabetes mellitus.
Answer 4
Visual impairment in DR may result from macular oedema or proliferative disease causing vitreous hemorrhage and/or tractional retinal detachment.
Diabetic macular oedema occurs as a result of the accumulation of extracellular fluid within the retina, causing disruption of the retinal architecture and interfering with retinal photoreceptor function. The condition known as 'clinically significant macular oedema' (CSMO) is a clinical diagnosis based on the area of thickening and its distance from the fovea. Clinically significant macular oedema can occur in both nonproliferative and proliferative DR.
In proliferative disease, abnormal fragile blood vessels grow from the retina and often use the vitreous strands as scaffolding to grow into the vitreous. These fragile vessels may bleed causing a vitreous hemorrhage. Alternatively they may form fibrous bands and 'pull' off the retina causing a tractional retinal detachment.
Patients with diabetes are also at an increased risk of cataract at a younger age. Cataracts in these patients are often of the posterior subcapsular type, which tend to impinge on the visual axis causing faster visual decline. In addition, erratic glycaemic control in patients with diabetes may cause transient visual blurring as a result of changes in refractive power from lens swelling.7
A decline in visual acuity is often the first indicator of the severity of ocular pathology, and it is important to test vision in each eye separately, both with and without the use of a pinhole. Failure of the vision to improve with the use of a pinhole is often an indicator of a potentially serious underlying cause.
Answer 5
The mainstay of the treatment of proliferative diabetic retinopathy remains pan-retinal photocoagulation (PRP) using an retinal laser.8 The rationale is to ablate the peripheral ischaemic retinal tissue to reduce the hypoxic drive (and therefore VEGF production) within the eye. The aim is to prevent severe visual loss by maintaining central vision where possible.
Vitrectomy surgery is performed in cases of slowly resolving vitreous hemorrhage and tractional retinal detachment. In some cases, vitrectomy may be performed early in vitreous hemorrhage to clear the blood and remove tractional bands, followed by completion of the retinal laser.
The current standard of care for the treatment of clinically significant macular oedema is focal or grid retinal laser, which has been shown to reduce the risk of moderate visual loss by at least 50%.9 Extreme care must be taken not to laser too closely to the fovea.
In recent years, increasing evidence has demonstrated the effectiveness of anti-VEGF intravitreal injections such as bevacizumab10 and ranibizumab11,12 in the treatment of diabetic macular oedema. A study of ranibizumab 0.5 mg combined with either prompt or deferred laser therapy was significantly more effective than laser alone in improving vision in patients with macular oedema at 2 year follow up.13 Anti-VEGF treatments are not currently available on the Pharmaceutical Benefits Scheme for the treatment of diabetic macular oedema, but may be increasingly used in the future.
Answer 6
In all diabetic patients, long term glycaemic control is necessary to reduce the risk of microvascular complications including retinopathy. A large, prospective study from the United Kingdom demonstrated the benefit of tight glycaemic control in reducing the risk of DR in type 2 diabetes.14 In addition, blood pressure control was shown to be paramount.14
Management of other cardiovascular risk factors, including hypercholesterolaemia, is also important. Fenofibrates in particular may be of benefit in the management of DR.15 Without adequate control of these risk factors, local ophthalmic treatment is likely to be of limited benefit.
Noncompliance with medication and treatment is a major issue in patients with DR, especially in those with advanced disease. It must be emphasised to patients with advanced DR that the aim of treatment is to prevent severe visual loss, and that without adequate treatment (both systemic and ophthalmic), the disease is likely to continue to progress.
Henry had a history of noncompliance with medications and medical appointments. His deteriorating vision was the reason for his re-engagement with the health system on this occasion. Importantly, his GP now has an opportunity to work with Henry, together with the multidisciplinary health team, to deliver a unified message regarding the importance of long term compliance with treatment.
Conflict of interest: none declared.