Cardiovascular disease (CVD) is a major cause of death in Australia, killing one person every 12 minutes and accounting for nearly 30% of all deaths in Australia in 2014.1 Direct healthcare expenditure for CVD exceeds that for any other disease group.2 Elevated levels of risk factors for CVD (ie blood pressure, blood cholesterol levels, diabetes status, smoking status, obesity, physical inactivity) at all ages are associated with a higher lifetime risk for CVD mortality.3
Current guidelines support an absolute CVD risk (ACVDR) management approach,4 assessing the combined effects of multiple risk factors. This involves categorising individuals into low (<10%), moderate (10–15%) and high (>15%) risk of experiencing a cardiovascular event within a five‑year period,4 and tailoring therapeutic interventions accordingly. However, many general practitioners (GPs) continue to use an individual risk factor approach.5 A recent study found that nearly 70% of individuals at high risk of CVD are not receiving recommended blood pressure and lipid-lowering therapy.6
One way to approach this treatment gap may be through the use of automated electronic medical record (EMR)-based clinical decision support (CDS) tools.
Existing EMR-based CDS tools to assess ACVDR have to be manually deployed during patient consultation and require additional manual data entry by the practitioner to assess CVD risk. This can be a barrier to their use by adding an extra task to an already busy consultation. EMR-based CDS tools need to automatically provide clear, actionable and personalised care recommendations in real time to GPs and patients. This can support patient-centred, evidence-based management of CVD risk factors.7,8
The aim of this study was to develop an EMR-based CDS tool that is integrated with the medical software system. This eliminates the need for manual data entry, and provides personalised treatment recommendations and prompt discussion between the GP and patient.8
In this article, we briefly describe the development of the Treat to Target CVD (T3CVD) tool, and describe a pilot study undertaken to evaluate the acceptability and feasibility of the tool in Australian general practice.
Methods
Development of the T3CVD tool
Our aim was to create an EMR‑integrated CDS tool that implements a clear and comprehensive method of communication of ACVDR in a user-friendly, efficient platform. We drew on literature9 that suggests that EMR-based CDS tools should:10
- seamlessly provide treatment and management recommendations that are personalised to individual patients (including pharmacological and lifestyle treatments)
- include integrated risk equations and clinical algorithms to prioritise treatment suggestions on the basis of patient benefit and clinical outcome
- be displayed in a way to prompt discussion between the GP and the patient.
We collaborated with an academic health and biomedical informatics unit in the iterative development of the tool.
We chose the 1991 Framingham risk equation for the risk assessment to predict five-year absolute CVD,11 which is based on the recommendations of the National Vascular Disease Prevention Alliance guidelines4 and is familiar to GPs, being commonly used in medical record software. This risk equation was derived from a largely Caucasian population in Framingham, Massachusetts, US; however, evidence suggests that when tested with eight other international studies and absolute risk assessment methods, it reported equivalent or higher predictive ability than other methods.10
The T3CVD tool was designed to be automatically triggered by a set of deployment criteria (Figure 1), ensuring that the tool was targeted for patients with moderate-to-high ACVDR (>10%), and for whom an actionable risk factor existed. The aim of the T3CVD tool was to minimise interference in the clinical encounter.
The T3CVD tool was developed to work in conjunction with GRHANITE, a widely used software that securely extracts data from the EMR.9,12 GRHANITE interrogates the EMR for deployment criteria; if all criteria are satisfied, and if the calculated ACVDR is greater than 10%, the pop-up deploys (Figure 2). The pop-up displays ACVDR and possible risk reductions if modifications were made to smoking status, blood pressure and cholesterol levels.
Three questions for the GP were included in the tool (Figure 2) to assess its effectiveness in promoting communication and management changes around CVD risk.
In instances where a risk factor required for ACVDR is unavailable, an ‘Attention’ pop-up deploys to show the GP the missing risk factors. It also prompts GPs to state whether they would collect and/or record these factors to facilitate future calculations (Figure 3).
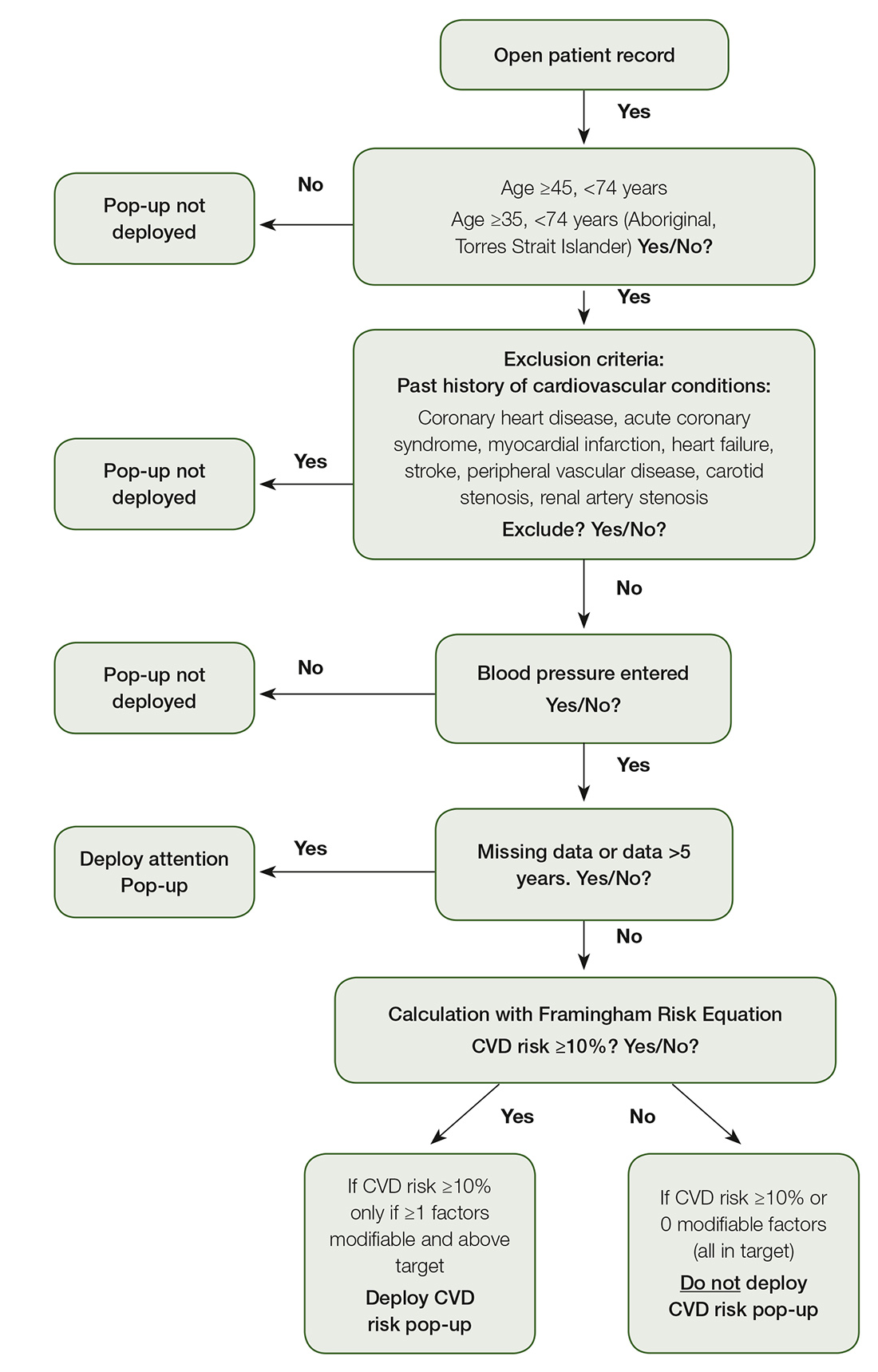
Figure 1. Deployment criteria
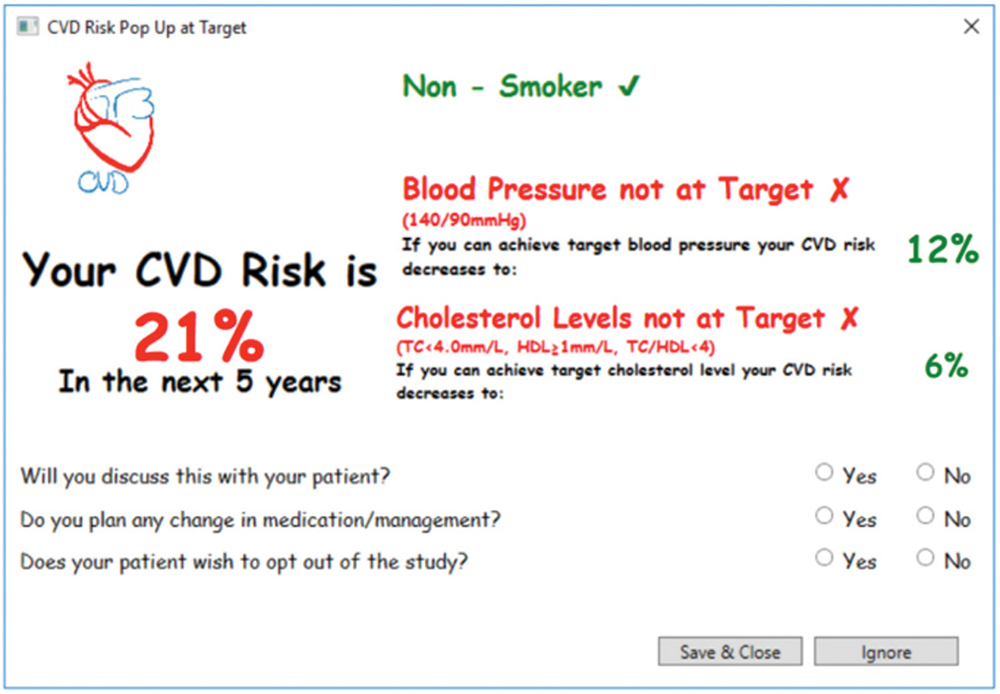
Figure 2. CVD risk pop-up
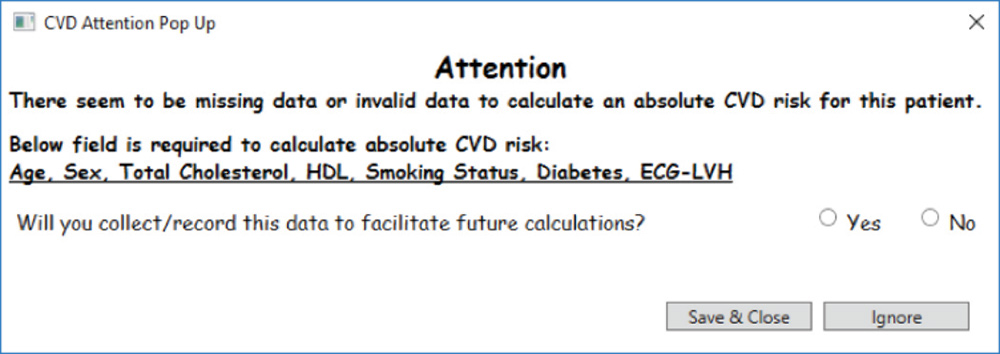
Figure 3. ‘Attention’ pop-up
Tool deployment
The T3CVD tool was piloted in one practice in metropolitan Melbourne, Victoria. Five GPs agreed to use the tool for one day in their routine practice.
Evaluation and analyses
We used a mixed-methods approach to evaluation. We collected clinical data, patient demographics, ACVDR and associated GP responses by extracting data from the EMR using GRHANITE. Descriptive statistics were used to characterise patient and GP responses.
At the end of the tool deployment period, we conducted semi-structured interviews with participating GPs, which focused on the acceptability and feasibility of the T3CVD tool. The interviews also focused on the GPs’ experiences with the tool in real-world clinical practice, prompting GPs with screenshots of the deployed tool (Figure 2). We used a framework analysis, which adapted a conceptual framework developed by the Institute for a Broadband-Enabled Society (IBES) for the evaluation of telehealth implementations.13 Three main themes that were considered were:
- patient control
- clinical quality of care
- technology capability/capacity.
The University of Melbourne’s Human Research Ethics Committee approved the study (ethics ID: 1647106). This study was conducted between May and August 2016.
Results
Five GPs from one general practice clinic took part in the pilot, using the T3CVD tool in their routine practice for one day. Thirty-nine patients were assessed with the tool; that is, they reached a point in the algorithm where the Framingham risk calculation was applied (Figure 1). The ACVDR pop-up was deployed for nine (23%) patients. Patient demographic and clinical data are presented in Table 1. The mean ACVDR of the sample was 15% (standard deviation [SD]: 4). The ‘Attention’ pop-up was deployed for 11 (28%) patients, identifying that they had invalid or missing data. The remaining 19 patients had an ACVDR that was too low to trigger the deployment of the tool.
Table 1. Demographics and clinical characteristics for patient
|
Patient characteristics
|
Patients assessed (n = 39)
|
Pop-up deployed (n = 9)
|
|---|
|
Demographic factors
|
|---|
|
Age (years), mean (SD)
|
56.0 (15.3)
|
65.8 (6.2)
|
|
Female, n (%)
|
16 (41.0)
|
1 (11.1)
|
|
Clinical factors
|
|---|
|
ACVDR (%), mean (SD)
|
–
|
15 (4)
|
|
Systolic blood pressure (mmHg), mean (SD)
|
134 (19)
|
155 (13)
|
|
Diastolic blood pressure (mmHg), mean (SD)
|
82 (11)
|
84 (9)
|
|
Total cholesterol level (mmol/L), mean (SD)
|
5.2 (1.2)
|
5.6 (1.4)
|
|
High-density lipoprotein (mmol/L), mean (SD)
|
1.6 (0.4)
|
1.4 (0.4)
|
|
Patients who smoked, n (%)
|
2 (5.1)
|
0 (0)
|
|
Patients with diabetes, n (%)
|
6 (15.4)
|
2 (22.2)
|
|
ACVDR, absolute cardiovascular disease risk; SD, standard deviation
|
GP participants indicated that they discussed the results with eight (88.9%) patients, and that medication or management changes to address this risk would be made for seven (77.8%) of the patients.
Among the 11 patients with missing or invalid data, GP participants indicated that they would collect data to facilitate future calculations with 10 (90.9%) patients. Cholesterol levels and smoking status were the two most common fields where there were missing or invalid data, comprising five (45.5%) out of the 11 patients.
Patient control
Overall, the GP participants indicated a broad acceptability of the T3CVD tool. Under the main theme of patient control, responses highlighted the way the tool might help to motivate patients to make behavioural changes to manage their ACVDR:
Well I think it would help people become motivated to make change. – GP3, female
The tool also helped GP participants to share decision-making around the choice of therapy with patients:
So that is usually when I am trying to convince someone to be on medication, so I am trying to convince [the patient] to be on either a hypertensive or statin or cholesterol-lowering agent, because they are not certain they need to be, so I always need to sort of show them where they sit in the risk. This would take away my need to do that. – GP3, female.
GP participants described how the ACVDR assessment pop-up necessitated additional time, often prompting the need to arrange a follow-up consultation. GP participants found ways to integrate the ACVDR risk tool into their workflow while supporting continuity of care:
I mean even if we already used up most of the consultation time, I still think you’ve always got to have a bit of time just to say ‘Look, you know there is a red flag that has been brought up here, we don’t necessarily have time to go completely into it today, but I need you to come back, you know, for us to further look into this’. – GP4, female.
Clinical quality of care
Within the theme of clinical quality of care, GP participants described the effectiveness of the T3CVD tool’s ease of use, automatic functionality, capacity to save time and link to established guidelines. The tool’s capacity to save time in the consultation through its automatic functionality was seen as an improvement over the current CVD risk tool found in the EMR software:
I think it is a great idea, makes it easier, quick and sort of automatic for this information to come up … because that is our problem, you just don’t have enough time, so if anything takes time, you’re not going to use it, you’re not going to make the very most of it. So it is very good. – GP3, female.
GP participants highlighted the tool’s capacity for clinical decision support by providing management guideline targets, helping them to decide what treatment should be given:
[The tool is] very relevant when you are trying to decide whether to treat cholesterol or blood pressure … I think it is very useful, a very useful tool and very user-friendly. – GP3, female.
GP participants noted the potential for the tool to be disruptive, as one of many pop-ups they needed to deal with:
I think the hardest thing with the pop-up tool is actually getting the balance right, so you’re not getting too many that it is really cluttered with stuff you don’t need to read. – GP5, female.
One GP participant wanted the information on the tool to be stored temporarily and returned after the primary focus of the consultation was addressed:
I was only annoyed when I couldn’t get rid of it and I did fiddle around with it and it did go, but having got rid of it, I’d just like it to store the information, in the consultation so I can come back to it. – GP2, male.
Technology capability/capacity
The theme of technology capability/capacity is related to the reliability, compatibility and availability of technical support for the tool. In this small study, budgetary restrictions affected the level of resource that could be devoted to the technical aspects of this proof-of-concept project. The development was taken to the alpha testing stage only. Environmental factors that can cause reliability issues (eg variations in operating systems) were encountered.
One GP participant emphasised that when trialling technology, such as the T3CVD tool, there must be excellent technical support:
We are trying a very new idea in general practice, looking at trialling technology tools, and we have to have really good support with both ends of the developer stuff. – GP1, male.
GP1 highlighted the need for testing in a real-world setting:
We have tested a simple issue, which is if you trial something and you don’t actually have a live test, with people watching it the whole time, then it doesn’t work. So clearly there is some sort of problem with the tool, the logic would be that the people that were trialling it should have been monitored by the people who made it to see it worked. – GP1, male.
Discussion
The findings of this study suggest that the T3CVD tool was able to act as a reminder for GPs to discuss ACVDR with patients. The majority of GP participants indicated that when the tool was deployed, they would discuss ACVDR with their patients and they intended to make management/medication changes to address this. The GP participants identified the tool’s capacity to improve efficiency and clinical care, indicating the broad acceptability of the tool in general practice. The GP participants also perceived that the T3CVD tool could be used to enhance patients’ motivation and participation, thereby reducing their ACVDR by working towards the risk factor targets.
The results suggest that the tool also supported GP continuity and longitudinal nature of care, consistent with the findings of Holbrook et al.14 Where the tool was deployed, GP participants found a way to integrate this into their workflow, at times prompting a return consultation. The tool also gave patients access to evidence-based information in a setting that was accessible and familiar.
Studies have documented that future CDS tools should better integrate with medical software systems, and the use of information from the EMR should be maximised to reduce data entry.15 The results demonstrate that the T3CVD tool addresses these issues with its automatic functionality. While we tested the system on only one medical software system, the GRHANITE data extraction software is agnostic and has the capacity to make the tool interoperable across multiple EMR software systems.
We identified important issues related to the feasibility of the tool’s use in real-world practice. Extensive resources are required to perform the level of real-world testing and developmental refinement required to take a proof-of-concept system such as this to a production stage.
The complexity of the EMR introduced barriers to the extraction of clinical data. For example, we were only able to accurately determine if a patient had a history of a cardiovascular condition if it had been coded in the EMR. Extracting the results of electrocardiograms in EMRs is problematic, so the tool had to make an assumption that patients did not have left ventricular hypertrophy when calculating their ACVDR. The use of free text search terms to support the extraction of past histories could minimise underestimation of the ACVDR. During the first deployment, there was an issue centred on data extraction from the EMR because of the complexity of the clinical information data storage in the EMR.
The findings of this pilot study align with the work of Peiris et al, which identified issues in accessing health and clinical data from disparate parts of the EMR, including free-text information.15 The accessibility of clinical data determines the tool’s ability to extract the required information and limit burdensome data entry for ACVDR calculations. Training for GPs to enter coded diagnoses rather than free-text diagnoses, and development of tools to clean and code existing data within the EMR, could assist with this. Requiring EMR software vendors to build in a required coded ‘reason for visit’ box prior to being able to prescribe, order a test, write a referral or conclude the consultation could also improve data quality.
Strengths of this pilot study include the real-world testing of the tool and the use of the established IBES framework to inform the evaluation. Limitations of this pilot study include the small number of participants, the restriction of the study to one site and the limited technical budget. The assumptions regarding left ventricular hypertrophy and difficulties extracting the history of CVD data mean that ACVDR may have been underestimated. The study only explored the perspective of the GPs; further work should explore patients’ views.
Conclusion
The T3CVD tool is the first tool to be used in Australian primary care that has the capacity to operate across multiple EMR systems. It demonstrates not only current CVD risk, but also risk reduction, possible with the implementation of lifestyle or pharmacological management. The findings suggest that the T3CVD tool was acceptable in general practice and has the capacity to support GPs in ACVDR management, and to encourage patient motivation and participation. Further investment in, and investigation of, the tool are indicated to improve the current management of CVD risk in general practice.
Authors
Jason Chiang BBiomed (Hons), PhD candidate, Department of General Practice and Primary Health Care, University of Melbourne, Vic. jason.chiang@unimelb.edu.au
John Furler MBBS, DipObst, DCH, PhD, FRACGP, Associate Professor, Department of General Practice and Primary Health Care, University of Melbourne, Vic
Douglas Boyle PGDip IT, BSc (Hons), PhD, FACHI, Associate Professor, Department of General Practice and Primary Health Care, University of Melbourne, Vic
Malcolm Clark MBBCh, BAO, LRCP&SI (NUI), FRACGP, Honorary Clinical Senior Fellow, Department of General Practice and Primary Health Care, University of Melbourne, Vic
Jo-Anne Manski-Nankervis BSc (Hons), MBBS (Hons), PhD, FRACGP, Lecturer – Primary Care, Department of General Practice and Primary Health Care, University of Melbourne, Vic
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.