Skin conditions are a quotidian component of the workload in general practice, accounting for approximately 15% of presentations.1 Of these presentations, up to one‑third involve the diagnosis and treatment of a benign or malignant skin neoplasm.2,3 In global terms, Australia has a high incidence of skin cancer, with a lifetime risk of non-melanoma skin cancer in the order of 70%, and an age-standardised incidence almost an order of magnitude greater than in other countries.4 Many of these lesions require confirmation of the diagnosis with histopathological examination.
Once a clinical decision to perform a biopsy has been made, the approach will vary depending on the potential differential diagnoses, as well as whether the intent of the biopsy is for diagnosis only, or diagnosis and complete excision of the lesion. While it is difficult to make generalisations about an optimal approach, we will focus on a number of broad principles that apply to most situations, and highlight approaches to some specific common lesions. As always, if there is ever any doubt, a phone call to the dermatopathologist is often the most efficient way to get advice, and provides an opportunity for collaborative discussions involving the case.
General principles
Biopsies of potentially neoplastic lesions may represent an attempt at excising the entire lesion (often with the intent of achieving diagnosis and definitive treatment in a single procedure), or may represent a partial biopsy intended for diagnostic purposes only, with definitive treatment to follow once the nature of the lesion is established. It is critical for the reporting pathologist to be aware of which of these two broad categories a specimen belongs. It is often difficult to determine this from the histological sections alone. This information also affects the approach to macroscopic assessment and the selection of sections for microscopic examination. Whereas clinicians will often use a punch biopsy as a convenient means to excise small lesions in their entirety, histopathologists generally assume that a punch biopsy specimen represents a partial sample, unless otherwise indicated. Figure 1 illustrates the standard terminology regarding the clinical intent of any biopsy specimen, and using these terms on the pathology request form should be routine practice.
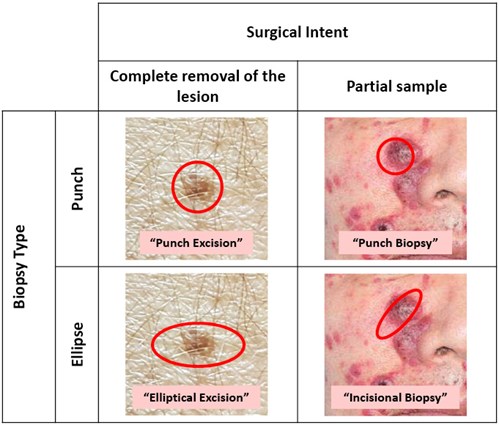
Figure 1. The nature of the biopsy, in particular, whether it represents an attempt at complete excision or a partial biopsy, should be clearly communicated to the pathologist to avoid confusion. The correct terminology is illustrated above
A biopsy intended to excise a lesion in its entirety would typically be expected to include a comment regarding margin status. Clinicians should be aware that because of the cylindrical nature of punch biopsy specimens, it is impossible for the pathologist to examine the entire circumferential margin, and the plane of sectioning (and hence margins assessed) will be essentially random for a punch ‘excision’. Elliptical excisions allow for the closest margins (typically along the sides of the ellipse) to be selectively examined (Figure 2). In some cases, it is valuable to mark one pole of an ellipse with a suture to indicate the orientation of the specimen on the request form (eg ‘suture marks the 12 o’clock pole’). This allows any involved margin to be specified (eg ‘the carcinoma extends to the 9 o’clock margin’).
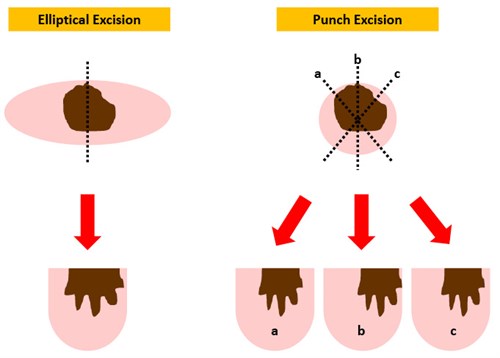
Figure 2. The limitations of margin assessment on a punch excision should be appreciated. For an elliptical excision specimen, the lesion can usually be identified clearly and the closest margins (usually to the sides of the ellipse) can be directly sampled. However, punch excision specimens are usually bisected in a plane that is essentially random, as the small size of the specimen typically precludes an accurate macroscopic assessment of the margins. Thus, a positive or close margin can easily be missed if the plane of sectioning does not demonstrate it. In the above example, the positive margin seen in section b would have been missed by sectioning in other planes, such as a or c.
In addition to the usual demographic data and information on the precise anatomical location of the lesion, it is valuable to provide information regarding the clinical size, duration and progression of the lesion. In this regard, it is increasingly common to send good-quality clinical and dermatoscopic images with the pathology request form. For re-excisions or lesions with a prior biopsy, an indication of the original diagnosis, lesional extent and original margins are helpful. An indication of the lesion size is mandatory for punch biopsy specimens.
Where the biopsy represents a partial sample, it is intuitively obvious, but nevertheless worthy of repetition, that the representativeness of the specimen must be considered in conjunction with the clinical appearances. Neoplastic lesions frequently induce areas of reactive change that may be clinically inseparable from the neoplasm and are hence sampled in partial biopsy specimens. Many practitioners (at least one of the authors included) leave medical school with the impression that the optimal site to biopsy any lesion is at the edge, where the lesion interfaces with the surrounding skin. In fact, for neoplastic lesions, the best diagnostic yield is usually obtained from the centre of the lesion, thus avoiding the potentially confounding reactive changes around the edges. A notable exception to this is where the lesion shows evidence of central necrosis. In these instances, a more peripheral area may yield more viable lesional material.
Many neoplasms display heterogeneous histological appearances. For example, basal cell carcinomas (BCCs) frequently show mixed growth patterns, and 20–60% of melanomas show an associated component of melanocytic naevus.5–8 For these lesions, it is essential that the pathologist be aware of whether the entire lesion is available for examination so that any potential heterogeneity can be considered part of the final histological interpretation.
Actinic keratosis
The majority of actinic keratoses are readily recognised clinically. Actinic keratoses are widely regarded as precursor lesions for cutaneous squamous cell carcinoma (SCC). While many cutaneous SCCs are associated with actinic keratosis, it has been difficult to determine the percentage of all actinic keratoses that will progress to invasive SCC, with estimates ranging from less than 1% to 16%.9,10 The presence of ‘thickness’ on palpation, bleeding, rapid growth, pain or failure of standard topical treatments may prompt concern regarding the development of an invasive lesion and will typically lead to a biopsy. Actinic keratosis with ‘severe’ or ‘full thickness’ histological atypia (ie bowenoid actinic keratosis or squamous carcinoma in situ) shows significant clinical overlap with conventional actinic keratosis; similar observations regarding biopsy apply.
The histological distinction of actinic keratosis from an invasive SCC relies on visualisation of the deep aspect of the lesion, particularly its interface with the underlying dermis. Typically, this can be evaluated with adequate shave or punch biopsies, or elliptical excision. More superficial shave biopsies may not include this critical landmark, particularly in the setting of lesions thickened by overlying hyperkeratosis (hyperkeratotic actinic keratoses, particularly on the dorsal forearm and hand, frequently raise clinical concern for invasive carcinoma). Superficial shave biopsy may confirm the presence of a dysplastic process, but if the underlying dermis has not been adequately sampled, it will not be possible for the pathologist to exclude an invasive carcinoma. One study identified invasive SCC in 20% of cases of actinic keratosis transected by shave biopsy that subsequently underwent re-excision.11 While this rate is likely to have been inflated by selection bias, the study serves to highlight the potential for underdiagnosis of SCC with superficial shave biopsy.
Seborrhoeic keratosis
Seborrhoeic keratoses are common, and frequently brought to the attention of the clinician by a concerned patient. In the large majority of cases, these lesions can be recognised clinically and reassurance represents adequate management. It is important to be aware that melanocytic lesions, including occasional examples of melanoma, can have a verruciform clinical appearance, mimicking seborrhoeic keratosis.12,13 The role of ablative treatments (eg electrodesiccation) is beyond the scope of this review. As the histological diagnosis of seborrhoeic keratosis is robust, partial biopsy (eg punch biopsy) can be of value in confirming the clinical impression, and shave biopsy or excision can be diagnostic and therapeutic.
Non-melanoma skin cancer
The most common forms of non-melanoma skin cancer are BCC and SCC. The approach to surgical and non-surgical management of these lesions is beyond the scope of this review. However, a few general observations may be of value.
If diagnostic sampling is undertaken prior to definitive management, a small punch biopsy from the central portion of a lesion clinically suspected to be BCC or SCC is preferable. This avoids inducing a larger area of surrounding erythema associated with shave biopsy, and leaves the margins of the lesion intact, allowing for easier visualisation if definitive excision is required.14 In addition, areas of more aggressive infiltrative growth, which might direct a more aggressive surgical approach, are more likely to be located at the central invasive front of a tumour.
While there is a rationale for avoiding superficial sampling of central areas of ulceration and inflammation, this should not be taken to imply that peripheral sampling is necessarily preferable. In cases where central ulceration might hamper diagnosis, an incisional biopsy, including the central portion as well as more peripheral lesional tissue, may be appropriate. Many skin lesions induce a peripheral area of reactive epithelial hyperplasia, which appears to represent part of the neoplasm clinically, while other invasive tumours have a peripheral component of actinic keratosis or SCC in situ. Sampling of these areas can lead to a false negative diagnosis such as ‘benign hyperkeratosis’, or identification of only actinic keratosis. If a partial biopsy is being used, it should be noted that BCCs frequently display a mixture of growth patterns, and punch biopsy sampling will fail to identify an aggressive component in approximately 15% of cases.15 If the clinical examination suggests a superficial BCC or Bowen’s disease, a shave biopsy may be the most appropriate procedure. This is particularly the case for superficial BCC, as the neoplastic cells are typically discontinuous in two dimensions, thus punch biopsy samples may show only areas of superficial stromal reaction in some cases.
Keratoacanthoma
Keratoacanthoma is a controversial entity. While some authors consider it to be a subtype of SCC,16,17 most current classification schemes regard it as a separate entity with benign or low-grade biological behaviour.18 Clinically, keratoacanthoma typically presents as a flesh-coloured, dome-shaped nodule with a prominent central keratinous plug, with the characteristic history of rapid evolution.19 Strictly speaking, a true keratoacanthoma, if not excised, should involute over time. The problem is that there is significant histological overlap between the lesions that will regress (‘keratoacanthoma’), and those with persistent growth and metastatic potential (‘well-differentiated SCC’); thus, the distinction can be very subjective. Complicating the picture further are reports of frequent development of SCC within keratoacanthoma.20 Current clinical practice guidelines recommend treating keratoacanthoma as an SCC to mitigate these problems. From a practical standpoint, the diagnosis of keratoacanthoma is best made on a complete excisional biopsy specimen. If the clinical differential diagnoses lie between keratoacanthoma and SCC (as it usually does), it is our view that an unequivocal separation of these possibilities cannot be achieved on a partial sample, regardless of whether one interprets this as attributable to morphological overlap between the lesions or the potential for development of superimposed SCC.
Pigmented lesions
Australia and New Zealand have the highest incidence of melanoma in the world.22 In the setting of an atypical pigmented lesion, a biopsy is usually required to distinguish between a cutaneous melanoma and benign mimics, such as atypical naevi. Indeed, in the US, it is estimated that 1–2 million biopsies a year may be performed for this reason alone.23 Common pigmented skin lesions include:
- conditions with increased melanin pigmentation but no melanocytic proliferation; examples include ephelis, labial melanotic macule and post-inflammatory pigment alteration
- predominantly epithelial proliferations with increased pigmentation (eg solar lentigo, seborrhoeic keratosis), pigmented actinic keratosis and pigmented BCC
- melanocytic proliferations, such as lentigo simplex, melanocytic naevus and melanoma.
The Clinical practice guidelines for the management of melanoma in Australia and New Zealand recommend excisional biopsy with narrow (2 mm) clinical margins as the standard approach for biopsy of clinically concerning pigmented lesions.22 There are a number of reasons for this:
- Histological diagnosis of melanoma requires assessment of features such as size, symmetry and circumscription. On a punch biopsy sample, it is difficult to identify whether a lesion has been completely encompassed.24 When this is combined with a lack of clinical information on the size of the lesion or intent of management (eg partial sampling, excision), these critical morphological attributes are difficult or impossible to assess.
- Partial sampling, particularly punch biopsy sampling, is associated with an increased risk for underdiagnosis of melanoma. In one Australian study, the odds ratio for misdiagnosis was 16.6 with punch biopsy sampling, compared with excisional biopsies.25 It is worth noting that partial biopsy of melanocytic lesions is a recurrent cause of litigation.26
- Heterogeneity within melanocytic lesions is well recognised. Thus, lesions can show areas of conventional or dysplastic naevus, while other areas represent evolving melanoma.27,28 In addition, many melanomas contain areas of regression in which diagnostic changes of melanoma are absent (Figure 3).
- Breslow thickness, which guides management and is critical for prognostication, can only be definitively determined once the entire lesion has been examined.
- Many small melanocytic lesions, particularly ‘hypermelanotic’ lentiginous junctional naevi, which are frequently biopsied because melanin pigment in the stratum corneum leads to a dark clinical appearance, show architectural features that overlap with dysplastic naevi. In a small punch biopsy sample, these architectural features combine with appropriate diagnostic caution to cause concern for dysplastic naevus and recommendation for complete excision.
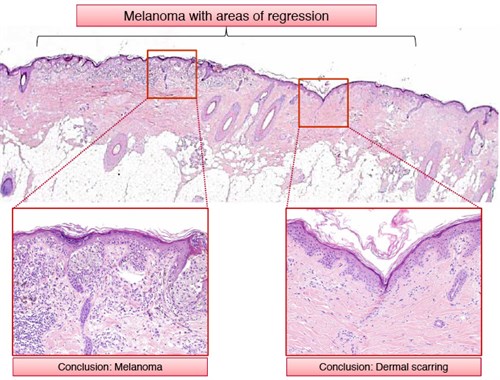
Figure 3. Melanocytic lesions are often heterogeneous; thus, partial biopsies carry a significant risk for misdiagnosis. In the above example, the lesion is a melanoma (in this instance melanoma in situ) with areas of regression. While a partial biopsy in some areas will yield the correct diagnosis, a biopsy inadvertently taken from an area of regression will yield a false negative result.
An argument can be made for ‘saucerisation’ shave biopsy (aiming for complete excision of the lesion), particularly for lesions with a relatively low clinical suspicion of melanoma on the upper trunk and proximal extremities, and which lack features suggestive of deeper dermal extension on palpation.29 Use of this technique appears to be most successful for practitioners who have the opportunity to regularly review the histological slides of specimens they have obtained and thus ‘calibrate’ their approach.
There are some circumstances in which excisional biopsy cannot be performed or is inappropriate, including:
- Broad lesions on the face: the differential diagnoses typically include lentigo maligna (melanoma in situ) and solar lentigo or macular seborrhoeic keratosis. After careful palpation to exclude an underlying component of invasive melanoma, a shave or incisional biopsy may be considered.29
- Large lesions at other sites and lesions on functionally sensitive sites (eg sole of the foot): punch biopsy or incisional biopsy to confirm a diagnosis prior to definitive management might be considered in these circumstances.
If, for whatever reason, a partial biopsy is performed for a pigmented lesion, it is mandatory to indicate this fact on the pathology request form. It is also mandatory to provide an indication of the lesion size, and preferably clinical and dermatoscopic images with the area of sampling marked. Clinicians should always remember that ‘a partial biopsy may result in a partial diagnosis, which may be a misdiagnosis’.26
There is clear evidence from multiple studies that clinicopathological correlation, including multidisciplinary review of clinical and dermatoscopic images in conjunction with the histology slides, can influence the diagnosis and management of patients with pigmented skin lesions.30 Where facilities for multidisciplinary input are available, they should be used, particularly in cases where there is an apparent discordance between clinical features and histological diagnosis.
Nail unit biopsy
Biopsy of the nail apparatus can be a useful adjunct for nail conditions that have escaped diagnosis via routine history, examination and microbiology. The technique requires an understanding of the nail unit anatomy, and for those unfamiliar with these procedures, consultation with or referral to a dermatologist or nail surgeon prior to biopsy is recommended.
The type of biopsies performed include excision, punch and longitudinal. The most appropriate biopsy depends on the site of the pathology within the nail unit and the risk of permanent damage from the procedure.31 It is important to orient the excision properly for the best result, and to communicate this orientation to the reporting pathologist, ideally by means of an annotated diagram or photograph. An excision in the nail bed is usually oriented longitudinally and a nail matrix excision is usually oriented horizontally.31 Pathological interpretation of a nail biopsy requires an understanding of the anatomy of the nail unit, including the histological characteristics of each component. These requirements are best met by a subspecialist dermatopathologist.
Conclusions
Biopsies of potentially neoplastic skin lesions are a necessity for general practice, especially in countries such as Australia with high rates of sun-related skin neoplasia. While the technical aspects of performing biopsies are familiar to most clinicians, the other considerations discussed in this article can be just as critical to maximising the chances of a correct diagnosis, as well as obtaining other critical information such as margin status.
Key points
- It is critical to communicate whether the biopsy represents a partial sample or an attempt at complete excision with clear margins. Where partial biopsies are submitted, information about the overall size of the lesion is critical to the pathological interpretation.
- In general, partial biopsies should sample the central portions of a lesion.
- Biopsies taken for the assessment of possible invasive SCC arising in actinic keratosis must include dermal tissue.
- A definitive distinction between a keratoacanthoma and SCC cannot be made on a partial biopsy.
- Excisional biopsy with narrow (2 mm) clinical margins is recommended as the standard approach for the biopsy of clinically concerning pigmented lesions. If this is not possible, the size of the lesion should be communicated to the pathologist.
- Punch biopsy is generally a poor modality for the diagnosis of melanocytic lesions.
Authors
Nathan Tobias Harvey, FRCPA, Consultant Pathologist, Department of Anatomical Pathology, PathWest, QEII Medical Centre, Perth, Western Australia; School of Medicine, University of Western Australia, Perth, WA
Jonathan Chan, FACD, Consultant Dermatologist, Department of Dermatology, Sir Charles Gairdner Hospital, Perth, WA
Benjamin Andrew Wood, Consultant Pathologist, Department of Anatomical Pathology, PathWest, QEII Medical Centre, Perth, Western Australia; School of Medicine, University of Western Australia, Perth, WA. benjamin.wood@health.wa.gov.au
Competing interest: None.
Provenance and peer review: Commissioned, externally peer reviewed.
Acknowledgement
The authors offer their thanks to Dr Nicole Swarbrick for reviewing this manuscript.