Dermatological conditions are a common component of the everyday workload in general practice, with approximately 15% of all patient presentations being related to a dermatological complaint.1–3 Although many of these presentations relate to a neoplastic process, a significant number will require the practitioner to consider non-neoplastic processes. This broad grouping of disorders (often informally referred to as ‘inflammatory’ skin disease) encompasses specific dermatoses (eg atopic eczema, psoriasis), as well as cutaneous infections, drug reactions, secondary processes (eg ulceration), cutaneous effects of primary diseases in other organs, and cutaneous manifestations of systemic diseases.
Many of these conditions can be readily diagnosed by a combination of clinical history and physical examination, with other laboratory investigations (eg serology, fungal scrapings) playing a role in a minority of cases. Nevertheless, biopsy for histopathology plays an important role in furthering the evaluation of patients in the following cases:
- when confirmation of a clinical diagnosis is required
- in challenging cases where specific diagnosis is hampered by overlapping clinical features
- in cases where the clinical presentation of a dermatosis is atypical and raises problematic differential diagnostic considerations.
Once a clinical decision to perform a biopsy has been made, it is critical to approach the procedure in a fashion that is most likely to yield diagnostically useful information. This avoids the inevitable frustration on the parts of the medical practitioner and patient when an invasive procedure has been performed for little apparent benefit. There is a wide range of clinical circumstances in which a skin biopsy may be performed, with innumerable potential differential diagnoses. Thus, it is difficult to make generalisations about an optimal approach that will be true in all cases.
In this review, we will focus on a number of broad principles that apply to most situations, as well as focus on specific scenarios that require a particular approach. As always, if there is ever any doubt, a phone call or referral to a dermatologist or dermatopathologist is often the most efficient way to get advice for a particular situation, and provides an opportunity for collaborative discussions involving the case.
General principles
Clinical information
The submission of a skin biopsy for histopathological examination is, in essence, a request for a specialist medical opinion and it should be treated as such. Appropriate clinical information, including details of the presenting complaint, previous medical history and a full list of medications, would be considered appropriate for any other patient referral, and so it should be for histopathology referrals. It is a widely held misconception that a pathologist can ‘read’ a skin biopsy without background clinical information, similarly to a biochemical analyser ‘reading’ a serum sodium level. The truth is that the histopathological interpretation of inflammatory skin disorders is, at best, limited without knowledge of the clinical context. Pathologists typically approach a skin biopsy by identifying a ‘tissue reaction pattern’ (eg spongiotic dermatitis, interface dermatitis, panniculitis). These tissue reaction patterns are each associated with a list of differential diagnoses and, by reference to the clinical information, the pathologist is able to see where the clinical and pathological differential diagnoses overlap. In addition to the usual information as to the age of the patient, gender and precise anatomical location, three points of information deserve specific attention.
Description of the lesions
A description of the duration and appearance of the lesions is critical. The use of standard dermatological terminology is very helpful here. This allows for unambiguous communication between the referring clinician and pathologist, and establishes an appropriate framework within which the dermatopathologist can categorise the pathological processes that are apparent microscopically. Table 1 outlines common dermatological terms used to describe skin conditions.
Table 1. Commonly used descriptive terms in dermatology
|
Dermatological Term
|
Description
|
|---|
|
Macule
|
A flat lesion (non-palpable), measuring less than 1 cm in maximum diameter, which may be either hypopigmented or hyperpigmented, or show other colouration
|
|
Patch
|
A flat lesion (non-palpable), measuring greater than 1 cm in maximum diameter, which may be either hypopigmented or hyperpigmented or show other colouration
|
|
Papule
|
An elevated (palpable), circumscribed lesion, measuring less than 1 cm in maximum diameter
|
|
Plaque
|
An elevated (palpable), circumscribed lesion, measuring greater than 1 cm in maximum diameter
|
|
Nodule
|
An elevated (palpable), circumscribed lesion with a greater volume than a papule, often measuring greater than 2 cm in maximum diameter. The lesion may extend into the subcutis
|
|
Vesicle
|
An elevated (palpable), circumscribed lesion filled with fluid (typically serous), measuring less than 1 cm in maximum diameter
|
|
Bulla
|
An elevated (palpable), circumscribed lesion filled with fluid (typically serous), measuring greater than 1 cm in maximum diameter
|
|
Pustule
|
An elevated (palpable), circumscribed lesion filled with purulent material, usually measuring less than 1 cm in maximum diameter
|
|
Crust
|
Dried serum, blood or purulent material on the surface of a lesion
|
|
Scale
|
A build-up of excess keratin on the surface of a lesion
|
|
Fissure
|
A linear cleft in the skin
|
|
Erosion
|
Partial thickness loss of the epidermis.
|
|
Ulcer
|
Full-thickness loss of the epidermis, with variable loss of deeper tissue
|
|
Excoriation
|
Scratch-related injury to the skin
|
|
Atrophy
|
Thinning of the epidermis or loss of dermal collagen, leading to wrinkling, ‘shininess’ or a depression on the surface
|
|
Lichenification
|
Thickening of the skin with accentuation of natural skin lines
|
Attention should be given not only to the primary features of the skin condition (eg distribution, size, shape, border configuration, colour, presentation as macules, papules, vesicles), but also to any secondary changes that may be present (eg erosions, excoriations, lichenification). In addition, a history of any extracutaneous symptoms (eg fevers, arthralgia) or family history (eg psoriasis) may be useful. As an example, a clinical history of ‘skin rash on the back’ does little to refine the diagnostic possibilities. Instead, a more fulsome description of ‘one-year history of itchy hyperpigmented patch over the medial scapular border’ immediately alerts the dermatopathologist to possibilities, including notalgia paraesthetica and/or macular amyloidosis.
A clinical photograph of the lesion(s) can be extremely valuable as an adjunct to a thorough morphological description. In this era of widespread availability of digital photography and ease of sharing, good-quality clinical photographs are becoming increasingly easy to obtain and should be forwarded to the pathologist wherever possible. In many cases, the clinical evolution of the disease has been documented by the patient themselves.
Clinical differential diagnoses
Accurate clinical differential diagnoses are invaluable in the optimal histological assessment of inflammatory dermatoses. As mentioned previously, many dermatoses that have distinctly different histological appearances overlap clinically and vice versa.
For example, hypertrophic lichen planus and lichen simplex chronicus may present with bilateral lichenified plaques on the legs, and the distinction of these entities may present a clinical challenge. However, as these conditions are each characterised by a different microscopic tissue reaction pattern, the distinction histologically is relatively straightforward. If the specific question is asked, ‘Is this lichen planus or lichen simplex chronicus?’, a clear result can be forthcoming. In the absence of such a question, the pathologist may be restricted to reporting a ‘lichenoid interface dermatitis’, which could be associated with dozens of specific disease processes. Conversely, if an inappropriately limited clinical differential diagnosis is offered, a biopsy may be interpreted as ‘in keeping with’ a proffered clinical suggestion, leading the unwary to inaccurately exclude other diagnoses that might show similar findings.
Medical and medication history
A detailed history of the patient’s medical condition(s) will often be of value in interpreting skin biopsy findings. Many cutaneous reactions are associated with systemic medical conditions, including infectious disorders (eg erythema multiforme associated with Mycoplasma pneumoniae infection) and connective tissue diseases (eg skin eruptions associated with systemic lupus erythematosus, neutrophilic vasculitis associated with rheumatoid arthritis), among others. Any history of internal malignancy is particularly important as cutaneous manifestations can include cutaneous metastases, malignancy‑associated genodermatoses, carcinogen-induced skin conditions, side effects of therapy and a diverse range of more indirect ‘paraneoplastic’ syndromes (eg paraneoplastic pemphigus, Sweet’s syndrome).
A medication history is also important, particularly if the introduction of a new medication seems to follow a temporal relationship with the appearance of the cutaneous lesions. Cutaneous eruptions are a common type of drug reaction, and the wide variety of reactions that have been described occupies extensive chapters in textbooks of dermatology and dermatopathology. The frequency of different drug reaction patterns varies for different medications – almost any drug can cause a skin reaction, but not all drugs can cause all skin reaction patterns. In many cases, drug reactions occur soon after exposure to a new drug (eg exanthematous or morbilliform drug reaction),5 but it is worth knowing that some reactions can occur after prolonged exposure (eg interstitial granulomatous drug reaction can develop after years of exposure).6 It should be remembered that non-prescription ‘medications’ can also be implicated in cutaneous reactions, including herbal preparations and other dietary supplements.7 In occasional cases, relevant occupational or family history may be of value.
We have, on occasion, heard comments to the effect that there is ‘not enough space’ provided on pathology request forms to allow for all this information to be provided. While we have sympathy for this view, we urge practitioners to not feel restricted by the amount of space provided on a standard request form. Modern computerised medical record systems make it relatively easy for the clinical record of a presentation, including past medical history and medications, to be printed and attached to a pathology request, as they would be for other specialist referrals.
Timing and site of biopsy
It is difficult to generalise about the optimal timing and location of a biopsy, which will vary depending on the clinical appearances and differential diagnoses.8 For obvious reasons, it is necessary to balance optimal diagnostic sampling with cosmetic and technical considerations when selecting a biopsy site. However, some general principles can be applied.
Age of the lesion
It is generally better to avoid lesions that show obvious secondary changes, such as excoriation. In many active inflammatory processes, and particularly in intensely pruritic conditions, this necessitates biopsy of early lesions. In blistering diseases, attention should be directed to newly formed or evolving blisters, rather than areas of erosion after a lesion has broken down. Alternatively, in some diseases, it is better to biopsy an established lesion (eg a scaly plaque of plaque psoriasis, a longstanding active lesion of cutaneous lupus erythematosus) as early lesions may not show specific diagnostic features. If the differential diagnoses include chronic fibrosing processes (eg lichen sclerosus, morphoea), biopsy of longstanding lesions is appropriate.
Prior treatment
Treatment (eg topical steroids) can markedly alter the histological appearances of many lesions. Where possible, attention should be directed to areas that are untreated or have been free of treatment for a period (preferably one month) prior to biopsy. If topical treatment has already commenced, an inconspicuous representative area can be marked, and the patient instructed to keep this site ‘steroid-free’ for as long as possible (eg one week) prior to biopsy.
Vasculitis
For the histological diagnosis of suspected small vessel vasculitis, biopsy of a purpuric lesion of 48–72 hours duration without secondary changes is ideal. Conversely, sampling for immunofluorescence is better performed on newly developed (<24 hours) lesions.
As with timing, the optimal site of sampling varies between different conditions, and is dependent on whether the sample is intended for immunofluorescence or light microscopy. For many active inflammatory conditions, a biopsy sample that includes the advancing edge of the lesion and established abnormal skin will be optimal. This should not be taken to imply that biopsies containing significant amounts of normal perilesional skin are required. Indeed, sampling of clinically normal skin at the expense of lesional tissue is generally unproductive for histological purposes (Figure 1). While there are exceptions to this rule, they are uncommon, comprising subtle disorders where quantitative differences from the adjacent normal skin are diagnostically important (eg circumscribed acral hypokeratosis, atrophoderma of Pasini and Pierini).
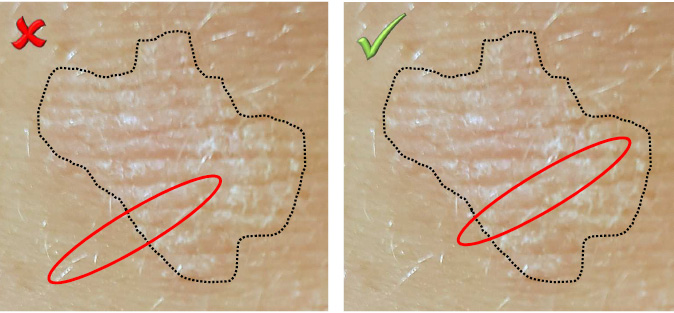
Figure 1. Positioning an incisional biopsy
When sampling the advancing edge of a lesion, significant amounts of normal tissue should not be taken at the expense of lesional material
Biopsy technique
The details of surgical techniques for biopsy are extensively covered in standard references and will not be reiterated in this article. Rather, some general points regarding the selection of a biopsy technique to optimise diagnosis will be made. Perhaps the most critical point is that the selection of biopsy technique must be predicated on an understanding of the anatomical distribution of the pathological findings in the differential diagnoses under consideration (Figure 2).9 In general terms, the less invasive biopsy techniques (eg shave biopsy, punch biopsy) are less likely to adequately sample deeper tissues, and are thus inappropriate for conditions where the pathological changes are located in deeper areas. From this principle a number of specific guidelines can be inferred.
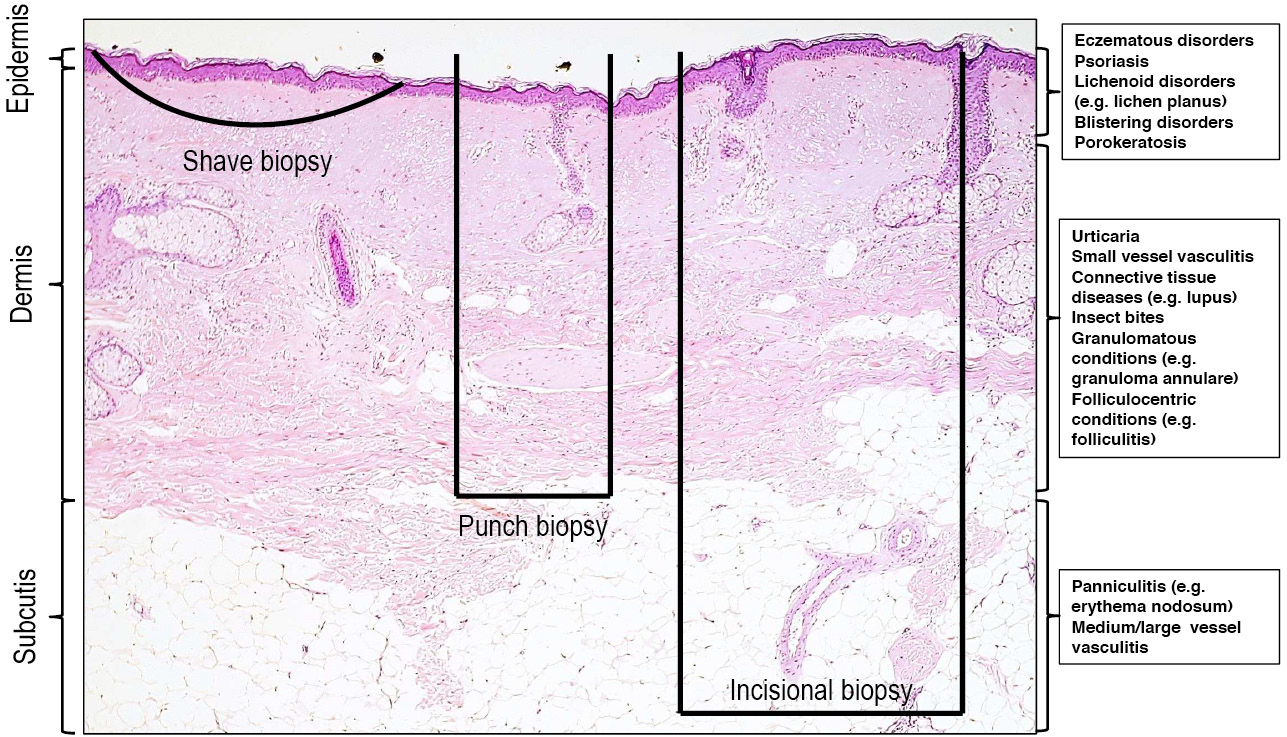
Figure 2. Sampling achieved with different biopsy types
The selection of biopsy technique must be predicated on an understanding of the anatomical layers that are typically sampled with shave, punch and incisional biopsy techniques, as well as the distribution of histopathological findings in the differential diagnoses under consideration
Panniculitis
Incisional biopsies including the subcutis (ie fat) are necessary if the differential diagnosis includes a panniculitis (eg erythema nodosum). Punch biopsy samples are typically inadequate and sampling of the subcutis by conventional punch biopsy is particularly unreliable when there is inflammatory damage to the panniculus.
Vasculitis
Incisional biopsies including subcutis are necessary for the assessment of medium or large vessel vasculitis, as the vessels typically affected in these conditions are not present in the superficial dermis. It is important to recall that the occluded vessel in retiform purpura is typically found at the deep dermal−subcutaneous interface in the pale centre of the lesion rather than at the reddened periphery.
Porokeratosis
If porokeratosis is suspected, sampling of the marginal ‘thread-like’ scale is necessary as this structure corresponds to the microscopic presence of cornoid lamellae, which is the hallmark of this diagnosis.
Annular lesions
Diagnosis of annular lesions (eg granuloma annulare, actinic granuloma of O’Brien) is most easily made on an incisional biopsy that includes the advancing edge of the lesion. The use of an incisional biopsy allows for the dermal changes (granulomatous inflammation in the two provided examples) to be visible. For these samples, it is prudent to suggest on the request form that the specimen be sectioned longitudinally, as unguided scientific/junior medical staff will typically section ellipses transversely, following the pattern used for the more common scenario of an elliptical excision.
Blistering conditions
Sampling of an intact blister is required for histological assessment of vesiculobullous disorders. Other than for small vesicles, this will typically require a deep shave, incisional or excisional technique, rather than a punch biopsy, as the epidermis will frequently be separated from the specimen with the latter.
Alopecia
Biopsies for alopecia require special techniques and laboratory handling, and for those unfamiliar with these procedures, consultation with a dermatologist or dermatopathologist prior to biopsy is recommended.
Material for ancillary testing
The most commonly used ancillary tests in dermatopathology are direct immunofluorescence (DIF), microbiological culture, flow cytometry and polymerase chain reaction (PCR) analysis for lymphocyte clonality. Many of these tests require separate material that has not been fixed in formalin. If in doubt, contact with the laboratory prior to biopsy is necessary.
Sampling for immunofluorescence should particularly be considered in the diagnosis of blistering disorders (eg bullous pemphigoid), connective tissue disease (eg cutaneous lupus erythematosus) and if Henoch-Schönlein purpura is being considered in the differential diagnosis of a presumptive small vessel vasculitis. Samples for DIF testing can be submitted in specialised solutions (eg Michel’s transport medium) or in gauze soaked in normal saline. Rapid transport to the laboratory is important, as the ability to reach a diagnosis declines 24–48 hours after biopsy. If biopsies are taken at ‘critical times’ (eg Friday afternoon), a phone call to the laboratory is recommended. The optimal sample for DIF testing for suspected bullous pemphigoid is perilesional skin from the trunk within approximately 10 mm of a bulla.10 Conversely, lesional skin should be selected for connective tissue diseases.9 DIF testing in cases of suspected vasculitis is best performed on newly developed lesions (<24 hours).
If an infectious aetiology is suspected, consideration should be given to submission of a separate, fresh sample to allow for formal microbiological studies. If organisms with specific growth conditions (eg fungi or Mycobacteria) are included in the differential diagnoses, these should be specifically mentioned on the request form to ensure appropriate handling on receipt at the laboratory.
If there is a suspicion of cutaneous lymphoma, clinicians are encouraged to liaise with the dermatopathologist prior to biopsy to determine appropriate sampling and triage of tissue for ancillary studies.
Clinicopathological correlation
In many cases, a discussion with a dermatologist or dermatopathologist can lead to diagnostic resolution of a problematic biopsy. In a small number of cases, a specific diagnosis may remain elusive. In this circumstance, it is often possible to develop an appropriate strategy for management, follow-up and consideration of further biopsy at a later point, on the basis of combined consideration of the clinical and histological findings.
Conclusions
A biopsy can be of great value in the assessment of a patient with a non-typical, progressive or otherwise clinically challenging skin condition. While the technical aspects of performing biopsies would be familiar to most clinicians, the other considerations as discussed in this article can be just as critical to maximising the chances of a firm diagnosis, or at least limiting the potential differential diagnoses.
Key points
- Detailed clinical description, submission of clinical photographs and focused clinical differential diagnoses will increase the diagnostic value of skin biopsy for inflammatory conditions.
- Selection of biopsy site and technique should be predicated on an understanding of the microanatomical distribution of the changes under differential diagnostic consideration.
- In many problematic cases, discussion with a dermatologist and dermatopathologist will facilitate a specific diagnosis or development of an appropriate approach to cases that remain enigmatic.
We have attempted to summarise an approach in Figure 3. We hope that this will provide a guide for clinicians undertaking skin biopsies.
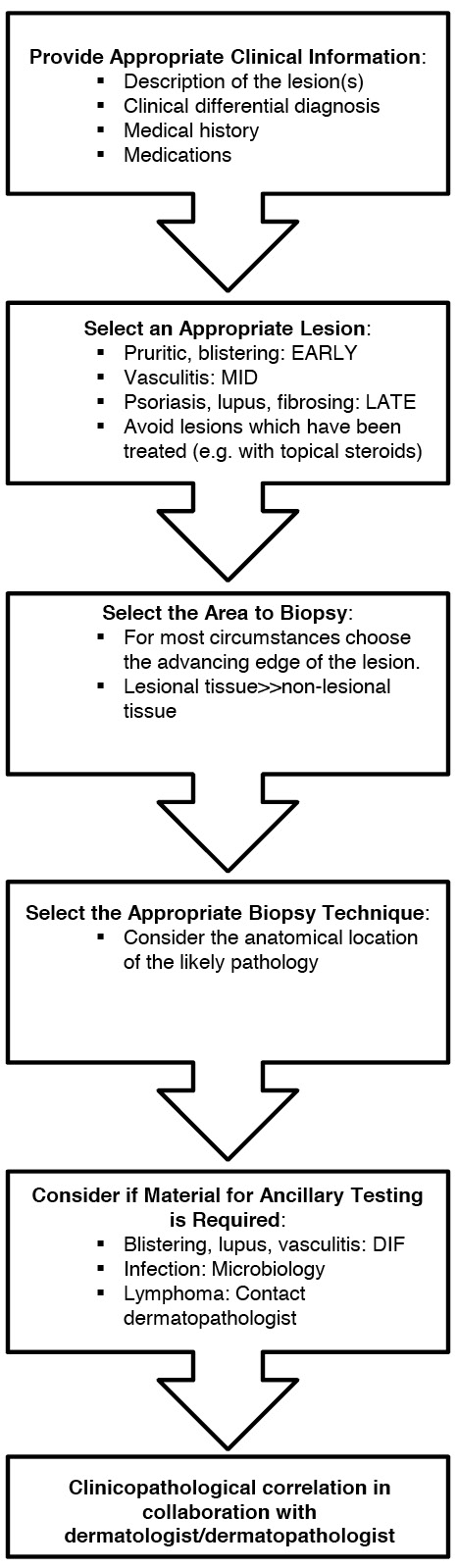
Figure 3. Flowchart summarising the approach to biopsy of an inflammatory skin condition
Authors
Nathan Tobias Harvey, FRCPA,Consultant Pathologist, Department of Anatomical Pathology, PathWest, QEII Medical Centre, Perth, Western Australia; School of Medicine, University of Western Australia, Perth, WA
Jonathan Chan, FACD, Consultant Dermatologist, Department of Dermatology, Sir Charles Gairdner Hospital, Perth, WA
Benjamin Andrew Wood, FRCPA, Consultant Pathologist, Department of Anatomical Pathology, PathWest, QEII Medical Centre, Perth, WA; School of Medicine, University of Western Australia, Perth, WA. benjamin.wood@health.wa.gov.au
Competing interest: None.
Provenance and peer review: Commissioned, externally peer reviewed.
Acknowledgement
The authors offer their thanks to Dr Nicole Swarbrick for reviewing this manuscript.