Scollypox’ is a colloquialism originating from the Gascoyne region (north-west) of Western Australia. It refers to suppurative skin lesions frequently acquired by commercial fishermen working on trawlers operating in the coastal regions (Figure 1). The term ‘scolly’ is local slang for scallops, which represent an important source of income for the local fishing industry. Fish packers, recreational fishermen, surfers, swimmers and tourists also frequently present with lesions sustained in the marine environment via coral cuts and abrasions, fish, mammal and crustacean contact injuries, filleting knife lacerations, penetrating hook injuries, or contamination of a pre-existing wound by sea water.
There are anecdotal reports from the Gascoyne region of scollypox resulting in severe skin infections with significant morbidity and mortality. Patient factors are known to play a significant part in the development of skin and soft tissue infections. Hence, the high prevalence of diabetes, obesity, liver disease, cardiovascular disease and alcoholism in the region,1 coupled with higher rates of intravenous drug use among fisheries workers,2 means the local population is at particular risk of acquiring marine-associated skin infections with the potential for considerable pathogenicity.3,4 It therefore remains paramount that there is a high index of suspicion for the possibility that such a wound contains marine organisms, so that appropriate antibiotic therapy is administered in a timely fashion.
Most infections sustained in a marine environment are caused by common skin commensals such as Group A Streptococcus and Staphylococcus aureus.5 Other pathogens are known to exist naturally in water (Pseudomonas species, Serratia species, Vibrio species, Aeromonas species, Erysipelothrix species and nontuberculous Mycobacteria species),6–8 while others are episodically washed into the waterways and oceans from the soil (Enterobacter species, Bacillus species, Actinomyces species and Klebsiella species).3,9,10
The Therapeutic Guidelines acknowledges that treatment of water-related infections is difficult and advises seeking expert advice.11 It is recommended that mild infections are treated empirically as for early cellulitis and erysipelas; however, treatment advice for specific water-related organisms depends on the water source.11
Current antibiotic guidelines for such infections are largely derived from reports and research performed in the US. This would seem a sensible undertaking; however, marine microbiology is itself determined by the conditions of the water – organic matter content, pH, temperature, ambient light, salinity, oxygen content, seasons and land-based rainfall, and run‑off.12 With the extensive biodiversity of the Australian coastline, particularly the Gascoyne bioregion given the Ningaloo reef and influences of the Leeuwin current (Figure 2), the marine organisms and their respective antibiotic susceptibility profiles may not be reflective of the remainder of the Australian marine environment or elsewhere.
The purpose of the study was to isolate pathological organisms involved with marine-associated skin and soft tissue infections in the Gascoyne region. By identifying the antibiotic susceptibility profile of the pathological organisms, we could compare these findings to the Therapeutic Guidelines and review the appropriateness of the advised empirical therapy in our local situation.
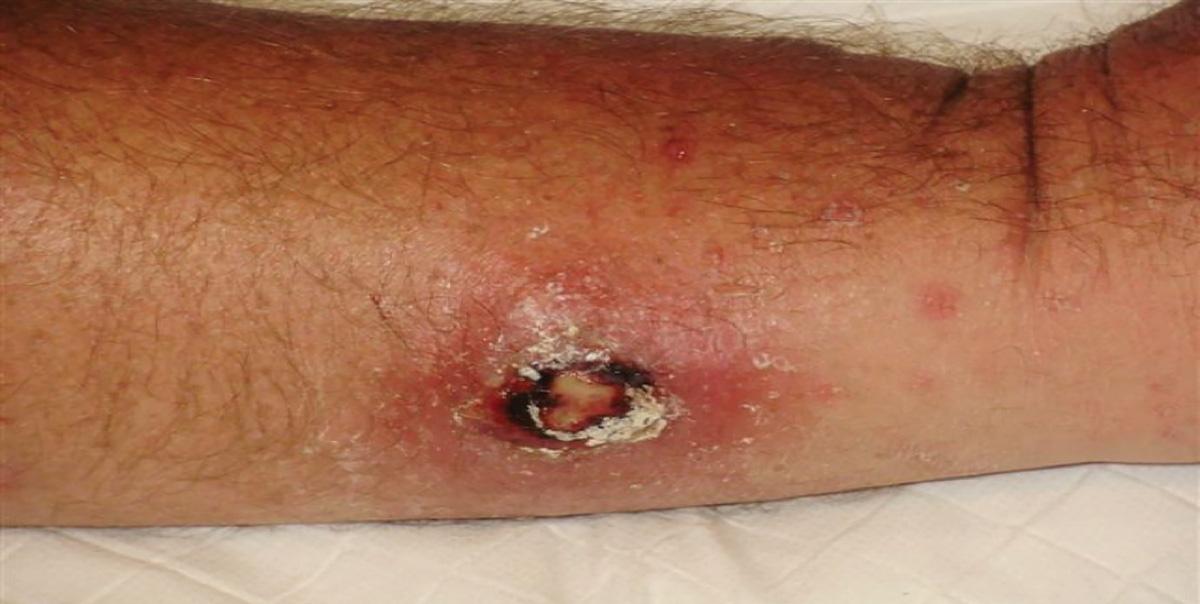
Figure 1. Classic marine wound infection
The image shows a classic marine wound infection on the forearm (flexor surface) of a commercial fisherman operating off the Gascoyne coastline. Photograph by Dr Andy Foote
Figure 2. Mid-west region of Western Australia. Image courtesy of WA Primary Health Alliance
Method
All doctors from the emergency departments of Carnarvon and Exmouth hospitals were engaged to help identify suspected marine-associated skin and soft tissue infections in patients presenting from 1 May 2010 to 1 August 2013. To qualify for inclusion in the study, patients had to have suppurating lesions from which a wound swab could be obtained. Injuries sustained in river environments were excluded. A single swab was obtained from each patient.
Potential participants were provided with an information sheet detailing the purpose of the study, signed a consent form, and had demographic details and fishing history collected. If the participant was a child, a parent or guardian provided consent.
Wound swab microbiology, culture and sensitivity
A swab was collected from the suppurating marine wound and sent to the regional laboratory, which performed microbiology testing. A pre-completed wound swab microscopy, culture and sensitivity (MCS) form stipulated that the wound swab was for suspected marine organism infection. All swabs were cultured on the four standard media: blood agar, colistin–nalidixic acid blood agar, MacConkey agar and laked blood agar with gentamicin. Two additional plates – thiosulphate-citrate-bile salts-sucrose agar and Ashdown’s medium – were used in this study to allow for easier identification of less common pathogens associated with water-related infections.
Ethics approval
Ethics approval for the study was received from the human research ethics committees of the Western Australia Country Health Service (reference number: HRAC2010:22) and the University of Western Australia (reference number: RA/4/1/4227).
Results
Twenty-eight patients were recruited. The average age of participants was 35.8 years (range: 7–65 years). The majority were male (78.6%) and involved with the commercial fishing industry (57.1% versus 42.9% for recreational fishers). Approximately 85% of infected wounds were on the hands or feet, and 10 patients had more than one wound. Nineteen patients had no other health issues, but eight had one and one had two comorbidities. Three wounds were sustained in the estuarine environment.
Patients who were systemically unwell when they presented, had significant comorbidities and/or deep infected lacerations were admitted to hospital (n = 10; 35.7%). They were treated with a combination of intravenous ceftriaxone, intravenous flucloxacillin or intravenous ciprofloxacin plus intravenous erythromycin or oral doxycycline while awaiting culture results. The remaining 18 patients were treated successfully on an outpatient basis.
The most common culture result was S. aureus, found in 64.3% (n = 18) of wound swabs. Methicillin-resistant strains of S. aureus (MRSA) made up 16.7% (n = 3) of the S. aureus cultured; these were collected later in the study, cultured from three out of the last seven swabs taken. The next most frequent culture result was Vibrio species in 32.2% (n = 8) of the wound swabs. Shewanella species were cultured from 14.3% (n = 4), Pseudomonas species from 14.3% (n = 4) and Group G Streptococcus from 10.7% (n = 3) of the wound swabs. There were no Aeromonas species isolated. Table 1 shows the culture and antibiotic susceptibility results by patient.
Polymicrobial infection was common, with more than one species of bacteria cultured from 39.3% of the swabs and more than two species from 17.8%. There were no deaths within the cohort.
Table 1. Culture results by patient
|
Sex, age (years)
|
Type of fishing
|
Comorbidity
|
Cultured bacteria (antibiotic susceptibility)
|
|---|
|
M, 34
|
C
|
None
|
Staphylococcus aureus (flucloxacillin)
|
|
M, 46
|
R
|
None
|
S. aureus (flucloxacillin)
|
|
F, 26
|
C
|
None
|
S. aureus (flucloxacillin)
|
|
M, 28
|
C
|
IVDU
|
S. aureus (flucloxacillin)
Group A Streptococcus (flucloxacillin, penicillin)
|
|
M, 65
|
R
|
IDDM
|
S. aureus (flucloxacillin, penicillin)
Vibrio alginolyticus (ceftriaxone, ciprofloxacin, gentamicin, doxycycline)
|
|
F, 27
|
C
|
None
|
S. aureus (flucloxacillin, clindamycin)
|
|
M, 24
|
C
|
None
|
S. aureus (flucloxacillin)
|
|
F, 12
|
R
|
None
|
S. aureus (flucloxacillin, gentamicin)
|
|
M, 37
|
C
|
IVDU
|
S. aureus (flucloxacillin, clindamycin, ciprofloxacin)
V. alginolyticus (ceftriaxone,ciprofloxacin, gentamicin, doxycycline)
Shewanella putrefaciens (ciprofloxacin, cotrimoxazole, gentamicin, meropenem, ticarcillin and clavulanate, piperacillin and tazobactam)
Pseudomonas aeruginosa* (ciprofloxacin, gentamicin, ticarcillin and clavulanate, ceftazidime)
|
|
M, 42
|
C
|
None
|
S. aureus (flucloxacillin)
V. alginolyticus (ceftriaxone, ciprofloxacin, gentamicin, doxycycline)
|
|
M, 53
|
C
|
Alcohol abuse
|
S. aureus (flucloxacillin, clindamycin, penicillin)
Group G Streptococcus (flucloxacillin, clindamycin, penicillin)
|
|
M, 49
|
C
|
Asthma
|
S. aureus (flucloxacillin)
V. alginolyticus (ceftriaxone, ciprofloxacin, gentamicin, doxycycline)
Group G Streptococcus (flucloxacillin, clindamycin, penicillin)
|
|
M, 57
|
C
|
Hep C
|
S. aureus (flucloxacillin, clindamycin, cotrimoxazole)
|
|
M, 34
|
C
|
IVDU, Hep C
|
S. aureus (flucloxacillin, clindamycin)
|
|
F, 19
|
R
|
None
|
S. aureus (flucloxacillin, clindamycin)
Group G Streptococcus (flucloxacillin, clindamycin, penicillin)
|
|
M, 52
|
C
|
None
|
MRSA (ciprofloxacin, clindamycin, doxycycline, cotrimoxazole, fusidic acid, rifampicin, mupirocin)
|
|
F, 23
|
C
|
None
|
MRSA (ciprofloxacin, clindamycin, doxycycline, cotrimoxazole, fusidic acid, rifampicin, mupirocin)
|
|
M, 50
|
C
|
None
|
MRSA (ciprofloxacin, clindamycin, doxycycline, cotrimoxazole, fusidic acid, rifampicin, mupirocin)
|
|
M, 26
|
R
|
None
|
V. alginolyticus (ceftriaxone, ciprofloxacin, gentamicin, doxycycline, piperacillin and tazobactam,
ticarcillin and clavulanate)
Pseudomonas stutzeri† (cefepime, ciprofloxacin, gentamicin, piperacillin and tazobactam,
ticarcillin and clavulanate)
Bacillus species† (amoxicillin, doxycycline, vancomycin)
|
|
M, 11
|
R
|
None
|
V. alginolyticus (ceftriaxone, ciprofloxacin, gentamicin, doxycycline)
|
|
M, 28
|
C
|
None
|
V. alginolyticus (ciprofloxacin, gentamicin, doxycycline)
S. putrefaciens (ciprofloxacin, gentamicin, ticarcillin and clavulanate, ceftazidime)
|
|
M, 38
|
R
|
None
|
Vibrio parahaemolyticus (ciprofloxacin, doxycycline)
Shewanella algae (cefepime, ciprofloxacin, gentamicin, piperacillin and tazobactam,
ticarcillin and clavulanate)
Group A Streptococcus
|
|
M, 49
|
R
|
None
|
Vibrio fluvialis (ceftriaxone, ciprofloxacin, gentamicin, doxycycline)
Shewanella algae (amoxicillin, ciprofloxacin, cotrimoxazole, gentamicin, doxycycline)
Providencia rettgeri (ciprofloxacin, cotrimoxazole, gentamicin)
|
|
F, 36
|
R
|
None
|
Group A Streptococcus (penicillin)
|
|
M, 33
|
C
|
IVDU
|
Group A Streptococcus (penicillin)
|
|
M, 63
|
R
|
NIDDM
|
P. aeruginosa* (ciprofloxacin, gentamicin,ticarcillin and clavulanate, ceftazidime)
|
|
M, 33
|
R
|
None
|
P. aeruginosa* (ciprofloxacin, gentamicin, ticarcillin and clavulanate, ceftazidime)
|
|
One patient (male, aged 7 years, recreational fisher, no comorbidities) did not have any positive cultures. Antibiotic resistances from microscopy, culture and sensitivity are available from the authors on request. *Pseudomonas aeruginosa may be a surface coloniser in the presence of an ulcer; †Pseudomonas stutzeri and Bacillus species may be environmental contaminants; C, commercial; F, female; Hep C, hepatitis C infection; IDDM, insulin-dependent diabetes mellitus; IVDU, intravenous drug use; M, male; NIDDM, non-insulin dependent diabetes mellitus; R, recreational
|
Discussion
This study confirms that bacteria cultured from marine-associated wounds in the Gascoyne region are similar to those detected in marine infections in regions with a similar range of coastal ocean temperatures.3,13–16 S. aureus was the most common bacterial species cultured, and Vibrio species were the second most common. More than one-third of the wound infections were polymicrobial.
Considering that the majority of wound swabs cultured S. aureus, the empirical use of flucloxacillin (or clindamycin if allergic to penicillin), as recommended by the Therapeutic Guidelines11 for treatment of cellulitis, was appropriate for this cohort. The three MRSA cultures were susceptible to ciprofloxacin, clindamycin and doxycycline.
In polymicrobial wound infections, it was common to culture organisms such as Vibrio species or Shewanella species in addition to S. aureus. These organisms were generally sensitive to doxycycline, so a combination of flucloxacillin plus doxycycline or clindamycin plus doxycycline appears to be a reasonable combination for empirical oral therapy on initial presentation in the Gascoyne. Vibrio species are not considered normal human flora, so their presence in wounds is presumed pathogenic; doxycycline is the empirical choice recommended by the Therapeutic Guidelines for water-associated Vibrio species infection.11
Marine-associated infections (particularly Vibrio species infections) can progress rapidly to fulminant sepsis, especially in patients with comorbidities, with a high mortality rate. Therefore, the decision to commence intravenous therapy should be made early.3,14,16,17
Alongside antibiotic therapy, the essential principles of meticulous wound care with aggressive irrigation and cleaning/debridement of wounds, using ultrasonography to search for foreign bodies such as fish spines or coral fragments, checking and updating of tetanus immunisation status, plus regular clinical follow-up and wound review are also important aspects of patient care.10,14,17–19
The local learning points for the region’s doctors were:
- Marine-associated infections are frequently polymicrobial infections.
- Empirical antibiotic therapy should include cover for S. aureus plus marine organisms, such as Vibrio species and Shewanella species, while waiting for wound swab MCS results.
- Wound swabs from suspected marine-associated infections need to be labelled accordingly as the laboratory uses additional culture plates to assist in easier identification of marine organisms.
Implications for general practice
- Marine skin infections can become life threatening quickly, so treat them seriously, especially if the patient has a comorbidity. Consider discussing with an expert early and referring for intravenous antibiotics early.
- Marine infections are often polymicrobial, with S. aureus and Vibrio species being the most commonly isolated in our cases.
- Take a wound swab if the lesion is suppurating and label it as a suspected marine infection.
- Use the Therapeutic Guidelines for initial antibiotic therapy and note that more than one antibiotic may be required.
Authors
Andy Foote MBChB, FRACGP, District Medical Officer, WA Country Health Service Gascoyne Region, WA; Senior Lecturer, Rural Clinical School of Western Australia, University of Western Australia, WA. Andrew.Foote@health.wa.gov.au
Robert Henderson MBBS, Radiology Registrar, Sir Charles Gairdner Hospital, Perth, WA
Andrew Lindberg MBBS, Anaesthetic Registrar, Royal North Shore Hospital, NSW
Carolyn Grigg MBBS, General Practice Registrar, Torquay Walk-in Clinic, Vic
Charlie Greenfield MBBS, MA, MD, PhD, FRCP, FRACP, SJOG, Physician, Medical Centre Geraldton, WA
Andrew B Kirke MBBS, BSc Hons, FRACGP, FACRRM, Senior Lecturer, Rural Clinical School of Western Australia, University of Western Australia, WA
Kirsten Auret MBBS, FRACP, FAChPM, Deputy Head of School, Rural Clinical School of Western Australia, University of Western Australia; Physician (Visiting Medical Practitioner), Western Australian Country Health Service – Great Southern, WA
Competing interests: None.
Provenance: Not commissioned, externally peer reviewed.
Acknowledgements
The authors would like to acknowledge the Rural Clinical School of Western Australia for support in the design and implementation of the study; medical staff of Carnarvon and Exmouth hospitals, Western Australian Country Health Services who assisted in gathering the data for this study; Dr Michael Leung, Clinical Microbiologist and Regional Microbiologist/Clinical Senior Lecturer, School of Pathology and Laboratory Medicine at the University of Western Australia; Dr Helen Van Gessel, Infectious Disease Specialist, Albany; Mr Luc Delhaize, Senior Scientist, Pathwest laboratory, Gascoyne Region; Sandy Van Houwelingen, Supervising Training and Development Officer, WA Fisheries Department; and Ray Bekeris, Master of the Fleet, NorWest Seafoods, Carnarvon, Western Australia.