Table 1. Common clinical features of pulmonary embolism2
- New or worsening breathlessness, particularly if it was sudden in onset
- Tachypnoea (respiratory rate of 20 breaths or more per minute)
- Chest pain, which may be pleuritic, or retrosternal and angina-like
- Tachycardia (heart rate > 100 beats per minute)
- Haemoptysis
- Syncope
- Hypotension (systolic blood pressure < 90 mmHg)
- Crepitations
|
Symptoms at presentation?
Less than 1% of patients with PE are asymptomatic, and at least one symptom of chest pain – sudden onset dyspnoea, fainting/syncope or haemoptysis – is present in 94% of patients with PE.3 Clinical features of PE have poor positive predictive value (PPV) when used alone.2 All the commonly used imaging tests utilise ionising radiation and have potential risks. PE rarely occurs in the absence of a risk factor (Table 2) and its likelihood increases progressively where multiple risk factors are present.4 A retrospective review of more than 2 000 subjects who underwent imaging with computed tomographic pulmonary angiography (CTPA) for suspected PE showed that there was a <1% chance of PE in the absence of the following risk factors: age over 65 years, immobilisation, malignancy, hypercoagulability, excess oestrogen or previous history of VTE.5
Table 2. Some risk factors for VTE4
| Strong risk factors (odds ratio >10) |
|---|
Major surgery
Major trauma (including hip or leg fracture)
Spinal injury
Hip or knee replacement |
| Moderate risk factors (odds radio 2–9) |
|---|
Knee arthroscopy
Central venous lines
Chemotherapy
Congestive heart failure or respiratory failure
Hormone-replacement therapy
Oral contraceptive therapy
Stroke
Pregnancy and puerperium
Previous VTE
Thrombophilia |
| Weak risk factors (odds ratio <2) |
|---|
Bed rest >3 days
Immobility due to sitting (eg. travel)
Increasing age
Laparoscopic surgery
Obesity
Varicose veins |
Who should be investigated?
Haemodynamically unstable patients with suspected PE should be immediately referred for consideration of thrombolysis in an inpatient setting as the risk of mortality is high (>15%).6
Figure 1 outlines a pathway for making decisions about when to image for suspected PE.
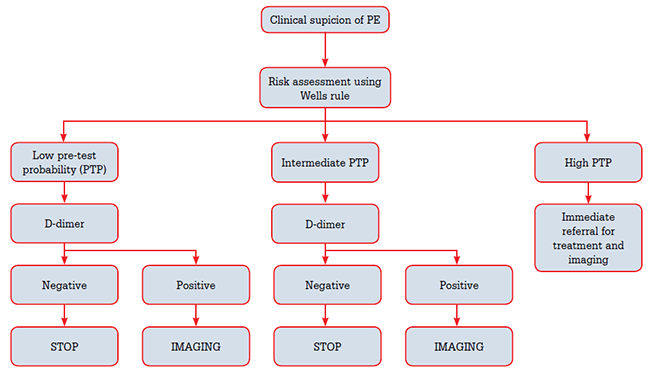
Figure 1. Suspicion of PE assessment pathway
Step 1. Assessing pre-test probability
Not all patients presenting with possible symptoms of PE need to undergo imaging tests and the interpretation of those tests depends on the pre-test likelihood of PE. A robust way to stratify patient risk is to use one of the validated clinical decision rules.7 Commonly applied tools are the Wells clinical decision rule8 or the revised Geneva rule9 (Table 3). Using the Wells system, a patient is stratified into low, intermediate or high probability, or alternatively into likely or unlikely. Relative prevalence of PE is 10% for low probability, 30% for intermediate probability and 65% for high probability groups.6 A high clinical probability should precipitate immediate referral for imaging6 and consideration of empirical low-molecular-weight heparin therapy if available as D-dimer testing cannot exclude PE in this group.6
Table 3. Clinical decision rules for PE: Revised Geneva9 and Wells score8
| Revised Geneva score9 | Wells score8 |
|---|
| Variable |
Points |
Variable |
Points |
| Predisposing factors |
|
Predisposing factors |
|
| Age >65 years |
+1 |
|
|
| Previous DVT or PE |
+3 |
Previous DVT or PE |
+1.5 |
| Surgery or fracture within 1 month |
+2 |
Recent surgery or immobilisation |
+1.5 |
| Active malignancy |
+2 |
Malignancy |
+1 |
| Symptoms |
|
Symptoms |
|
| Unilateral lower limb pain |
+3 |
|
|
| Haemoptysis |
+2 |
Haemoptysis |
+1 |
| Clinical signs |
|
Clinical signs |
|
| Heart rate |
|
Heart rate |
|
| 75–94 beats/min |
+3 |
>100 beats/min |
+1.5 |
| ≥95 beats/min |
+5 |
|
|
| Pain on lower-limb deep venous palpation and unilateral oedema |
+4 |
Clinical signs of DVT |
+3 |
| |
|
Clinical judgement |
|
| |
|
Alternative diagnosis less likely than PE |
+3 |
| Clinical probability | Total | Clinical probability (3 levels) | Total |
|---|
| Low |
0–3 |
Low |
0–1 |
| Intermediate |
4–10 |
Intermediate |
2–6 |
| High |
≥11 |
High |
≥7 |
Step 2. D-dimer testing
D-dimer is a degradation product of cross-linked fibrin and is elevated in plasma in the presence of clot because of the activation of coagulation and fibrinolysis. A negative D-dimer using a quantitative enzyme-linked immunoabsorbent assay (ELISA) has a sensitivity of >95% and effectively excludes PE in low- and intermediate-probability groups. Qualitative D-dimer tests are less reliable, but they have been used safely in the primary care setting with the Wells rule in excluding PE.10 D-dimer cannot be used to confirm PE as fibrin is also produced in cancer, inflammation, infection and necrosis.6 The combination of clinical assessment and D-dimer testing misses less than 2% of VTE in a general practice population.10
Step 3. Imaging
Patients in low and intermediate pre-test probability groups with positive D-dimer and patients with high pre-test probability should proceed to imaging.
Lower limb (compression) ultrasound
Compression ultrasound (US) has high sensitivity for detection of proximal deep vein thrombosis (DVT), which is the source of PE in 90% of patients.6 A positive lower limb US is present in 30–50% of patients with PE11,12 and is useful where tests using ionising radiation are less desirable, for example in pregnancy.
Ventilation-perfusion lung scintigraphy
Ventilation-perfusion lung scintigraphy (VQ) (Figure 2) uses macro-aggregated albumin (MAA) particles labelled with technetium-99m to assess lung tissue perfusion and compares it with ventilation images obtained after inspiring an aerosol of technetium-99m-labelled fine-carbon particles. In Australia, the patient is usually imaged with single photon emission computed tomography (SPECT), from which multiple reconstruction planes can be produced similar to CT. No specific preparation is required and there are no absolute contraindications. The test takes approximately 30 minutes and is usually well tolerated. Conventional planar imaging can be used for morbidly obese patients who exceed the system table weight limits or for patients who are unable to lie flat, but are less accurate than SPECT or CTPA.13
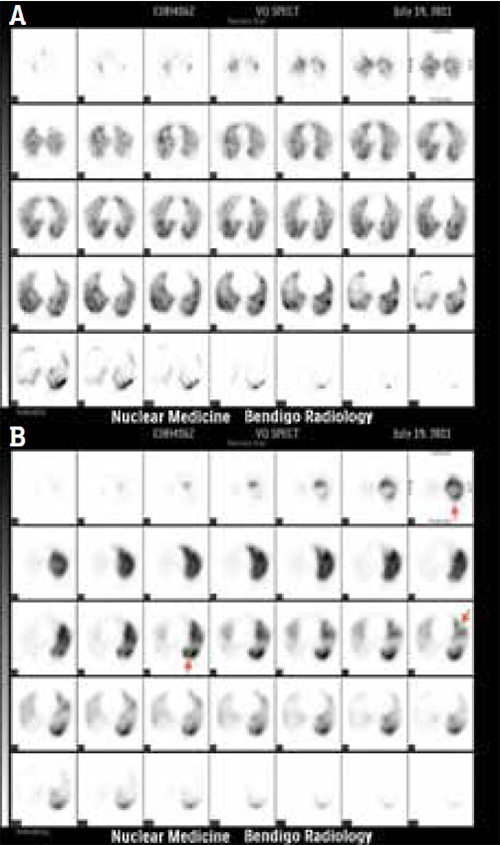
Figure 2a,b. VQ scan demonstrating PE
When PE occludes a pulmonary artery branch, the area supplied by this vessel will appear as a ‘cold’ defect with no activity on perfusion imaging and no corresponding defect on ventilation imaging, called a VQ ‘mismatch’. A ‘matched’ defect present on both ventilation and perfusion indicates hypoperfusion is due to vasoconstriction secondary to hypoventilation and not due to PE.
The Prospective Investigative Study of Acute Pulmonary Embolism Diagnosis (PISAPED) approach to perfusion scan interpretation defines PE “+” or present based on one or more wedge-shaped perfusion defects regardless of size. This system is easier to understand for referrers (it is similar to the binary system used in CTPA) and has high interobserver agreement. The PPV of PE + scan is 92% and increases to 99% when combined with high clinical pre-test probability.14 This approach also reduces the proportion of non-diagnostic scans.13,15–17
The use of tomographic techniques (SPECT) in VQ scintigraphy and the simplified perfusion scan diagnostic approach improves both sensitivity and specificity by detecting more subsegmental defects and has superior sensitivity when compared directly to 4-slice CT.13,15,18 99m-Tc-technegas also improves specificity and negative predictive value (NPV)19 and is routinely used in Australia, but is still unavailable in the United States. Many referring doctors in Australia are unaware of this and the negative effect it has had on diagnostic performance of the VQ scan in large international multicentre trials like the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) studies.
A normal VQ scan excludes PE and a positive scan in the presence of intermediate to high pre-test probability confirms it.6,17 Further investigation is required where clinical likelihood and imaging tests are discrepant or if the test is non-diagnostic.6
Radiation burden is very favourable (1.1–1.5 mSv) compared with CTPA and makes the VQ scan very useful in pregnancy and younger patients.6
Computed tomographic pulmonary angiography
CT is becoming the method of choice for evaluating pulmonary vessels because of its wider availability and ability to demonstrate alternative causes of symptoms. CTPA is fast and generally well tolerated (Figure 3). Most scanners can scan patients who weigh up to 180 kg albeit with reduced scan quality and higher administered radiation dose.
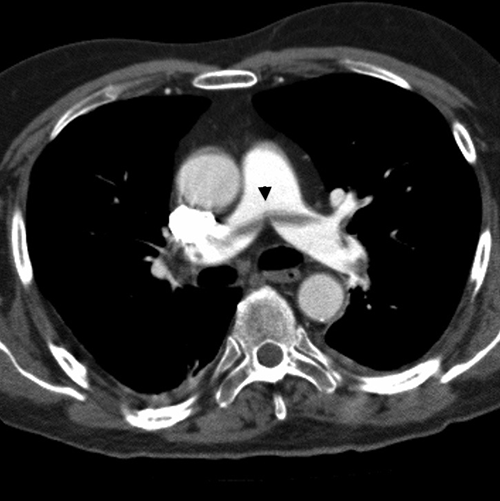
Figure 3. CTPA demonstrating PE
In the low and intermediate pre-test probability groups, a negative CTPA has a high NPV of 97% and 89%, respectively, when non-diagnostic scans are removed.20 The PPV of a positive test in the high pre-test probability group is above 90%.20 Discrepancies between clinical pre-test likelihood and CT result, such as a negative CT in the high pre-test probability group, should be followed with further imaging with lower limb US, VQ scan or pulmonary angiography to avoid under-treatment and associated risks.6,21(Figure 4)
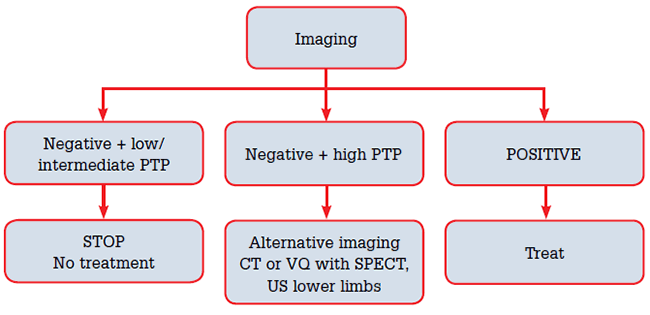
Figure 4. Managing imaging results
CT has superior sensitivity for the detection of small subsegmental emboli when compared with planar VQ imaging, but increased diagnosis has been associated with an increase in complications from anticoagulation without a decrease in mortality from PE.22–24 Small emboli are believed to be resorbed with no clinical effect.23
CT is relatively contraindicated in the presence of moderate-to-severe renal disease because of the risk of contrast-induced nephropathy. CT also has a relatively high radiation dose of 7 mSv (or more than 5 times a VQ scan). This makes it relatively less attractive for younger patients and young women in particular, because of the relatively large dose delivered to the radiosensitive breast. Novel CT reconstruction algorithms (so-called ‘low-dose’ CT), which allow less radiation to be delivered while preserving diagnostic quality are increasingly available, and should be used if available.25
VQ or CTPA?
Diagnostic accuracy of CTPA and VQ SPECT (using current criteria) is similar (Table 4) although CTPA detects clots in smaller vessels. CTPA may have the advantage of widespread availability where VQ scanning may not be available outside working hours. Radiation dose of VQ is significantly less than CTPA, which makes VQ preferable for young women and for follow up to establish baseline post-treatment. There is a higher risk of contrast-induced nephropathy with intravenous contrast for CTPA in patients with moderate-to-severe renal impairment and VQ is preferable in these patients.
Table 4. Comparison of VQ scan and CTPA17,20
| | Sensitivity
(95% CI) | Specificity
(95% CI) | Non-diagnostic | PPV | NPV |
|---|
| VQ using PISAPED |
80.5% (75.9–84.3%) |
96.6% (96.5–97.4%) |
0% |
84.7% |
94.5% |
| CTPA |
83%* |
96%* |
6% |
85.7% |
94.8% |
| * When non-diagnostic scans are excluded |
Pregnancy and breastfeeding
PE is a leading cause of maternal mortality. D-dimer assays are of limited usefulness, as D-dimer increases in the second trimester of pregnancy. Choice of imaging tests is controversial and should be discussed with a diagnostic imaging and/or nuclear medicine specialist. Some centres advocate CT over VQ because of a lower radiation dose to the foetus, particularly earlier in pregnancy. Perfusion-only lung scanning using a reduced dose of 100 MBq technetium-99m yields a dose to the foetus of approximately 0.25 mSv,26 which is identical to CTPA.27 The dose to the maternal breast is significantly less with VQ scans.28 Breastfeeding needs to be interrupted for 13 hours after VQ,26 but does not need to be stopped after CT contrast.
Conclusion
Exclusion of PE requires the combination of clinical assessment and appropriate use of diagnostic tests including D-dimer assay and imaging. Multidetector CT accessibility means that this is now often the diagnostic imaging test used, but VQ scanning is a well-validated investigation able to diagnose or eliminate PE with similar diagnostic certainty. An isolated subsegmental thrombus identified on CT is probably not significant and does not usually require treatment.
Provenance and peer review: Commissioned; externally peer reviewed.