Type 2 diabetes continues to be managed in general practice with limited but appropriate referral to specialists.3 General practitioners have access to several sets of guidelines published by various diabetes organisations to guide their management. These guidelines generally recommend a target HbA1c of less than 6% to 7%4 for newly diagnosed and uncomplicated patients. However, despite increasingly stringent guidelines for glycaemic control, over 60% of patients do not reach recommended glycaemic goals.4 In one Australian study, less than half (47.7%) of patients (n=3893) with type 2 diabetes seen in general practice had an HbA1c of <7.0% and 25% had an HbA1c of >8%.5
Clinical inertia
There are multiple reasons why the current management of glycaemia is falling significantly short of accepted treatment goals. Patient related factors include low diabetes health literacy resulting in poor adherence to treatments and/or goals for self management. A major GP related contributor is 'clinical inertia'.6 This has been described as 'recognition of the problem, but failure to act' or 'failure to initiate or intensify therapy appropriately'.7
A Canadian study of 243 GPs who completed records for nearly 2500 patients with type 2 diabetes found underutilisation of antidiabetic agents, with only 56% of patients with HbA1c above the target recommended for intensification of their treatment.6 The authors suggested that there was a gap between knowledge and practice. That is, although GPs are aware of guidelines on glycaemic control and recognise the need for intensifying therapy for poorly controlled patients, they need to be more aggressive in implementation.6
One Australian study found that among patients with type 2 diabetes, the median HbA1c at initiation of oral antidiabetic therapy and insulin was 7.7% and 9.4%, respectively.8 Another Australian study found that although GPs were likely to alter treatment regimens in patients with type 2 diabetes and established vascular disease and/or abnormal biochemical and physical parameters, they were more likely to underdose compared with specialists.3 The proportion of GPs who agreed with a panel of experts on the appropriate doses and choice of oral antidiabetic drugs was 20.2%.3 General practitioners were less likely than specialists to change treatment in patients who were on a sulphonylurea. The authors suggest this is because these agents have been available for many years, have a well understood mechanism of action, and are relatively well tolerated. They hypothesise that there is a reluctance to prescribe newer oral antidiabetic agents, such as dipeptidyl peptidase-4 (DPP-4) inhibitors or glucagon-like peptide-2 (GLP-2) analogues, but note that further research is necessary to identify the reasons for this.3
Why reaching HbA1c targets early is critical: the 'legacy effect'
The landmark United Kingdom Prospective Diabetes Study (UKPDS) offers valuable insights into the importance of early and tight glycaemic control. In this study, 3867 patients with a new diagnosis of type 2 diabetes were randomised to intensive therapy (either sulphonylurea or insulin) or conventional therapy with diet.9 Over the 10 year trial period, the median HbA1c was 7% in the intensive arm.9 This translates to a mean HbA1c of less than 7% in the first 5 years of the trial and an HbA1c above 7% for the remainder of the trial. The rates of myocardial infarction (MI) between intensive control and conventional therapy in the 10 years of the trial just missed statistical significance with a p value of 0.052. Diabetes related mortality and all cause mortality did not differ between the intensive therapy and conventional groups. However, the intensive arm experienced a significant reduction in microvascular outcomes.9 This finding was instrumental in defining the current HbA1c target of 7%.9
A 10 year follow up of UKPDS patients published in 2008 revealed that benefits of intensive intervention were sustained even when HbA1c worsened later on. The key point is that despite early loss of differences in HbA1c between the intensive and conventional therapy groups after completion of the original UKPDS trial, benefits in both macrovascular and microvascular endpoints persisted. This is the so-called 'legacy effect'.10 A similar phenomenon has also been described in patients with type 1 diabetes.11
In the intensive group, significant risk reductions for MI (15%, p=0.01) and death from any cause (13%, p=0.007) emerged in this follow up period over time, despite the lack of significant differences during the intervention phase, while microvascular risk reduction persisted (24%, p=0.001). In the metformin group, significant risk reductions persisted for any diabetes related endpoint (21%, p=0.01), MI (33%, p=0.005), and death from any cause (27%, p=0.002). Compared with overweight patients in the conventional therapy group, among a subgroup of overweight patients treated with metformin in the original trial, significant reductions in any diabetes related outcome, diabetes related death, MI, and all cause mortality were maintained at 10 year follow up.10
The UKPDS finding that early, tight glycaemic control results in improved long term outcomes is supported by a recent study aimed at determining 5 year mortality rates following type 2 diabetes diagnosis among patients with new onset disease seen within a few weeks of diagnosis.12 In this study, in addition to age at diagnosis, an important predictor of death in patients with recent onset of type 2 diabetes was the HbA1c achieved 3 months after diagnosis. Compared with patients with a 3 month HbA1c of <6.5%, mortality rate was almost doubled in patients with an HbA1c of ≥8.5, even after correcting for age at diagnosis (Figure 1).12
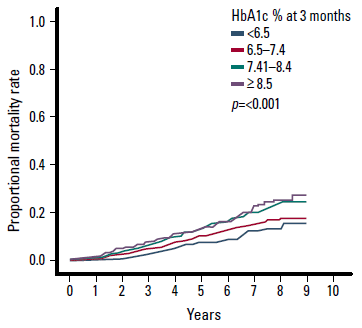
Figure 1. Mortality rates over 9 years corrected for HbA1c at 3 months after diagnosis of type 2 diabetes9
Adapted with permission from Kerr D, Partridge H, Knott J, Thomas PW. HbA1c 3 months after diagnosis predicts premature mortality in patients with new onset type 2 diabetes. Diabet Med 2011;28:1520–4
The ADVANCE and ACCORD studies
Two important large scale studies had somewhat different results than UKPDS. The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial assessed the effects on major vascular outcomes of lowering HbA1c to ≤6.5% in a broad cross section of patients with type 2 diabetes.13 An intensive glucose control strategy of a modified release gliclazide and other drugs as necessary lowered the average HbA1c to 6.5%, and was associated with a reduction in the incidence of the combined primary outcome of major macrovascular or microvascular events. However, the 10% relative reduction in the primary outcome was mainly due to a 21% relative reduction in the risk of new or worsening nephropathy. There was no reduction in macrovascular events. In addition, compared with standard control, an increased risk of severe hypoglycaemia and more hospitalisations were seen with intensive glucose control.13
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial was designed to determine whether a strategy of targeting normal HbA1c (ie. <6.0%) would reduce the risk of serious cardiovascular events in middle aged and elderly people with type 2 diabetes, HbA1c levels of ≥7.5%, and additional cardiovascular risk factors.14 The main finding of this trial was that, in a high risk population, 5 years of intensive therapy comprising multiple glucose lowering interventions designed to achieve an HbA1c <6% did not significantly lower the number of major cardiovascular events compared with targeting levels of 7.0–7.9%. In fact, the intensive approach appeared to result in more deaths.15
The ADVANCE and ACCORD trials enrolled high risk patients, who were on average 8 and 12 years older, respectively, than those in the UKPDS. These patients had been treated for 8 and 10 years, respectively, whereas those in the UKPDS were newly diagnosed and treatment naïve. Around 8% of patients in the UKPDS had a history of macrovascular disease, compared with about a third in the ADVANCE and ACCORD studies.10,13–15
In the ACCORD study, although the difference did not quite reach significance, there were more deaths among patients with HbA1c >8% at baseline compared with those with HbA1c of ≤8%.14 One interpretation of these findings is that if glycaemic control has been good since diagnosis, then intensive therapy is still beneficial. However, if prior glycaemic control is poor, it may be too late for intensive therapy late in the course of type 2 diabetes.
Implications for practice
The conservative stepwise approach to type 2 diabetes management involves a multidisciplinary team approach to lifestyle modification, followed by treatment with a single oral antidiabetic agent, often up titrated to maximal recommended doses before combination therapy is introduced. However, there is often a delay between stepping up from monotherapy (eg. metformin alone) to combination therapy (eg. metformin plus sulphonylurea) and this can result in unacceptable delays in achieving and maintaining glycaemic goals with the potential for long periods of hyperglycaemia. Such prolonged periods of hyperglycaemia should be avoided given the evidence that even short periods of hyperglycaemia increase the risk of micro- and macro-vascular complications.4
Thus, a more proactive approach, involving earlier use of combination therapy accompanied by diet and exercise reinforcement, is recommended.4 Such an approach acknowledges the importance of early achievement of glycaemic targets. In newly diagnosed patients, the goal should be to achieve an HbA1c of <6.5% within 6 months of diagnosis. If patients are not at goal after 3 months, combination therapy should be considered (Figure 2).4
As a patient's disease progresses and comorbidities/complications develop, the target HbA1c should be revisited. As the ACCORD results suggest, a target HbA1c of <6.0% cannot generally be recommended in patients who have a high risk of cardiovascular disease and longstanding, suboptimally controlled diabetes.15 The Australian Diabetes Society (ADS) recommends a general target of ≤7.0% for most patients with diabetes. However, the ADS notes that targets need to be individualised to a greater or lesser degree, with a target of ≤6.0% in some patients or up to ≤8.0% in others (Table 1).16 Similarly, the choice of therapy should be tailored to the patient's profile at initiation of treatment, looking at such factors as baseline HbA1c, duration of disease, the presence of complications/comorbidities, and the risk of hypoglycaemia.
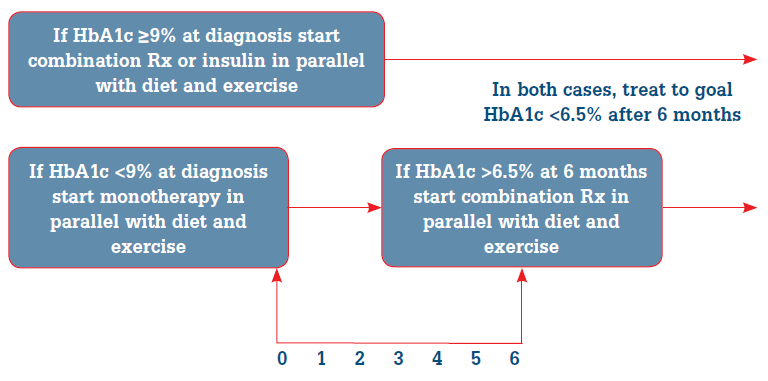
Figure 2. Algorithm for management of hyperglycaemia in newly diagnosed patients4
Adapted with permission from Del Prato S, Felton AM, Munro N, Nesto R, Zimmet P, Zinman B. Improving glucose management: ten steps to get more patients with type 2 diabetes to glycaemic goal. Int J Clin Pract 2005;59:1345–55
Table 1. Australian Diabetes Society target HbA1c ranges recommended for adults with type 2 diabetes
| General target ≤7% |
|---|
| Specific clinical situations |
- Diabetes of short duration and no clinical cardiovascular disease (CVD):
- requiring lifestyle modification and metformin ≤6%
- requiring any antidiabetic agents other than metformin and insulin <6.5%
- requiring insulin ≤7%
|
- Pregnant or planning pregnancy ≤6%
|
- Diabetes of longer duration or clinical CVD (any therapy) ≤7%
|
- Recurrent severe hypoglycaemia or hypoglycaemia unawareness (any therapy) ≤8%
|
- Major comorbidities likely to limit life expectancy (any therapy): symptomatic therapy of hyperglycaemia
|
| Adapted with permission from Cheung NW, Conn JJ, d’Emden MC, et al. Position statement of the Australian Diabetes Society: individualisation of glycated haemoglobin targets for adults with diabetes mellitus. Med J Aust 2009;191:339–44 |
Key points
- The prevalence of type 2 diabetes in Australia is on the rise.
- Despite increasingly stringent guidelines for glycaemic control, over 60% of patients with type 2 diabetes do not reach recommended glycaemic targets.
- Clinical inertia contributes to suboptimal glycaemic control.
- Evidence suggests that there is a legacy effect: that is, if patients achieve target HbA1c levels soon after diagnosis, they have better long term outcomes than those who do not reach target levels early, even if control is relaxed later in the course of disease.
- A proactive approach to treating type 2 diabetes is recommended: therapy should be individualised with early consideration of combination therapy and ongoing reinforcement of lifestyle modification messages.
- In newly diagnosed patients, the goal should be to achieve an HbA1c of <6.5% within 6 months of diagnosis. As a patient's disease progresses, the HbA1c target can be revisited in the light of comorbidities and complications.
Conflict of interest: All authors completed ICMJE conflict of interest forms. Ivan Kuo has received payment from AstraZeneca, Boehringer Ingleheim, Eli Lilly, Novartis, NovoNordisk, Sanofi Aventis, Servier and Bristol-Myer Squibb for lectures. Editorial support for the writing of this article was provided by a grant from AstraZeneca and Bristol-Myers Squibb.