There is little evidence that self-monitoring blood glucose (SMBG) benefits patients with non-insulin-treated type 2 diabetes (T2D). While considerably more expensive, SMBG appears to be no more effective than urine testing in improving glycaemic control.1 A recent meta-analysis found no convincing evidence to support routine SMBG in non-insulin-treated T2D.2
International Diabetes Federation (IDF) guidelines recommend using SMBG only where patients and/or health care providers have the knowledge, skills and willingness to incorporate SMBG and therapy adjustments into diabetes care plans.3 However, they acknowledge that some consequent benefit might be derived through educating patients to better understand their disease, engendering medication compliance and lifestyle changes3 that include healthy eating and weight loss. Any weight loss will often result in improved glycaemic, blood pressure and lipid profiles.4
While several studies have found little or no positive effect of SMBG in patients with non-insulin-treated T2D,5–7 including no reduction of patient body mass index (BMI),1,5,8 SMBG has been associated with lower quality of life,9 increased depression and anxiety,5,10 and higher costs, for no clinically significant improvement in other outcomes.9,11,12
Australian guidelines for diabetes management in general practice recommend less intensive self-monitoring for non-insulin-treated patients, but still suggest an ideal regime as before and after meals on 2–3 days per week for patients with stable glycaemic control.4
How commonly SMBG is currently used by Australians with non-insulin-treated T2D is not known. In 2012, the Pharmaceutical Benefits Advisory Committee (PBAC) commenced a review of medications and products used in diabetes management.13 Subsidised products including blood glucose (BG) testing strips are available through Diabetes Australia’s National Diabetes Services Scheme.14 The review objectives included describing SMBG utilisation and patterns of use for people with T2D, and determining clinical outcomes and benefits of SMBG relative to glycosylated haemoglobin (HbA1c) monitoring alone, for people with T2D who are not treated with insulin. The results of the review were not known at the time of preparing this paper.
The aims of this study were to determine the proportion of general practice patients with non-insulin-treated T2D who perform SMBG and to assess the association between SMBG and HbA1c levels, and BMI, to determine any measurable benefits of self-monitoring.
Methods
This investigation was conducted through a sub-study of the BEACH (Bettering the Evaluation and Care of Health) program. BEACH is a continuous, national, cross-sectional survey of Australian general practice activity. The BEACH methods are described in detail elsewhere,15 but in brief, each year we recruit approximately 1 000 randomly sampled, currently active recognised GPs. The GPs record details for 100 consecutive encounters with consenting, unidentified patients. Throughout the program, a series of sub-studies are carried out. Known as SAND (Supplementary Analysis of Nominated Data), these utilise the GP as an ‘expert interviewer’ to record, in discussion with the patient, aspects of patient health additional to the content of the encounter. The SAND investigations are therefore patient based rather than encounter based. For this SAND sub-study, 250 GPs were posted recording kits containing the SMBG questions, and 30 patients from each sample of 100 were surveyed over a 10-week period from 7th June to 15th August 2011.
GPs were asked to record for the patient: whether they had diagnosed T2D; their most recent HbA1c result; their current height and weight (for calculation of BMI); how often they measure fasting and/or post-prandial BG; and medications currently taken for management of BG levels.
We calculated proportions and robust 95% confidence intervals (CI) using survey procedures in SAS (version 9.2)16 that adjust for the study’s cluster design. Statistical significance of differences was judged by non-overlapping 95% CIs, which improve the interpretation of data because they provide robust upper and lower boundaries for the probable size of the true effect. Calculated BMI scores were compared for patients who self-monitor daily, self-monitor weekly or less, and never self-monitor. BMI scores of patients with T2D were also compared with those of all patients whose BMI was recorded at BEACH encounters in 2011–2012.17 Missing data were removed from the analyses.
An unadjusted prevalence estimate was calculated as the number of patients with T2D as a proportion of the total sample of respondents. This estimate can be interpreted as the prevalence of T2D among patients who present to GPs at any given time.
Results
Recording pads with completed SMBG questions for 5730 patients were returned by 194 GPs (77.6%). Figure 1 shows the age distribution of patients to be highly representative of that at the 104 million general practice encounters claimed across Australia through Medicare in 2011–2012.18
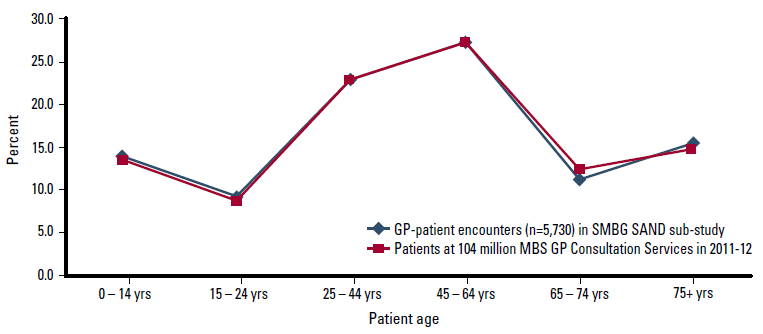
Figure 1: Age distribution of patients at GP-patient encounters – this SAND sub-study vs all GP consultation items claimed through Medicare in 2011–12
The prevalence of T2D was 8.1% (95% CI: 7.1–9.1; n=464). All T2D patients were aged 18 years or older. The age-specific rate increased with age, peaking at 20.2% of patients aged 65 years or older.
Current medications for BG management were recorded for 458 T2D patients. The majority (57.9%; n=265) were taking oral medication only, 10.7% (n=49) were on insulin plus oral medications, 5.2% (n=24) were on insulin only, and 26.2% (n=120) were not taking any medication. All further results are for the 385 non-insulin-treated T2D patients.
The prevalence of non-insulin-treated T2D was 6.7% (95% CI: 5.9–7.6; n=385). The mean HbA1c level for these patients was 7.1% (95% CI: 7.0–7.2) (54.1 mmol/mol).
Table 1. Patients with non-insulin-treated type 2 diabetes – self-monitoring blood glucose behaviour and HbA1c
| |
Patients |
Mean HbA1c |
| (n) |
%* |
%
(95% CI) |
mmol/mol
(95% CI) |
| All patients with non-insulin-treated T2 diabetes |
385 |
100.0 |
7.1
(7.0–7.2) |
54.1
(53.0–55.2) |
Patients who self-monitor BG (ever)
(missing = 36) |
278 |
79.7 |
7.1
(7.0–7.3) |
54.1
(53.0–56.3) |
Patients who self-monitor both fasting & post-prandial BG at least occasionally
(missing = 36) |
214 |
61.3 |
7.1
(7.0–7.3) |
54.1
(53.0–56.3) |
Patients who self-monitor either fasting or post-prandial BG at least occasionally
(missing = 36) |
64 |
18.3 |
7.1
(7.0–7.3) |
54.1
(53.0–56.3) |
Patients who never self-monitor BG
(missing = 36) |
71 |
20.3 |
6.7
(6.5–7.0) |
49.7
(47.5–53.0) |
| * Missing data removed so denominator = 349 |
Table 1 shows that testing routine was reported for 349 of the 385 patients with non-insulin-treated T2D, of whom 20.3% never self-monitored and 79.7% self-monitored fasting and/or post-prandial BG at least occasionally. Both fasting and post-prandial BG were monitored by 61.3%, either daily (14.6%), weekly (20.3%), less than once per week (13.2%), or monitored both but at different times (13.2%) (eg. fasting weekly, and post-prandial less than once weekly, etc.) (results not tabulated). Most patients self-monitored fasting BG (79.4%) and/or post-prandial BG (69.7%). Mean HbA1c was significantly higher among patients who tested daily (either fasting or post-prandial BG), compared to those who never tested (Table 2).
Table 2. Patients with non-insulin-treated type 2 diabetes – self-monitoring of fasting and post-prandial blood glucose
| |
Patients with non-insulin-treated T2 diabetes |
| |
(n) |
% of patients with T2D*
(n = 385) |
% of patients who self-monitor*
(n = 278) |
Mean HbA1c |
%
(95% CI) |
mmol/mol
(95% CI) |
| Fasting BG measurement (missing = 41) |
| Daily |
106 |
30.8 |
38.8 |
7.4
(7.1–7.7) |
57.4
(54.1–60.7) |
| Weekly |
104 |
30.2 |
38.1 |
6.9
(6.7–7.1) |
51.9
(49.7–54.1) |
| <1 per week |
63 |
18.3 |
23.1 |
7.1
(6.8–7.4) |
54.1
(50.8–57.4) |
| Total who self-monitor fasting BG |
273 |
79.4 |
100.0 |
7.2
(7.0–7.3) |
55.2
(53.0–56.3) |
| Never test fasting BG |
71 |
20.6 |
|
6.7
(6.5–7.0) |
49.7
(47.5–53.0) |
| Post-prandial BG measurement (missing = 71) |
| Daily |
60 |
19.1 |
27.4 |
7.5
(7.2–7.8) |
58.5
(55.2–61.7) |
| Weekly |
91 |
29.0 |
41.6 |
6.9
(6.7–7.2) |
51.9
(49.7-55.2) |
| <1 per week |
68 |
21.7 |
31.0 |
7.2
(6.9–7.5) |
55.2
(51.9–58.5) |
| Total who self-monitor post-prandial BG |
219 |
69.7 |
100.0 |
7.2
(7.0–7.3) |
55.2
(53.0–56.3) |
| Never test post-prandial BG |
95 |
30.3 |
|
6.8
(6.5–7.0) |
50.8
(47.5-53.0) |
| * missing data removed |
Calculation of BMI showed that 47.5% of patients with non-insulin-treated T2D were obese (Table 3). Compared with BMI of adult patients at BEACH encounters in 2010–2011, a significantly higher proportion of these patients were obese, and a significantly smaller proportion were normal/underweight. Similar proportions were overweight. The proportion of patients in each BMI category did not differ by BG testing status (Table 3).
Table 3. Body mass index – adult patients at BEACH encounters in 2010–11, and of patients with non-insulin treated type 2 diabetes by self-monitoring status
| Header |
BEACH sample 2010-11
% (95% CI)
(n = 31,315) |
Non-insulin treated T2 diabetes patients
% (95% CI)
(n = 334)* |
Patients who self-monitor daily
% (95% CI)
(n = 111) † |
Patients who self-monitor weekly or less
% (95% CI)
(n = 155)‡ |
Patients who never self-monitor
% (95% CI)
(n = 68)◊ |
| Obese |
26.7 |
(26.0–27.5) |
47.5 |
(41.7–53.3) |
46.8 |
(36.7–57.0) |
51.6 |
(43.9–59.4) |
44.1 |
(32.1–56.1) |
| Overweight |
35.1 |
(34.4–35.7) |
34.1 |
(28.8–39.4) |
35.1 |
(25.6–44.6) |
30.3 |
(23.2–37.5) |
38.2 |
(25.9–50.5) |
| Normal |
35.8 |
(35.0–36.7) |
18.1 |
(13.8–22.3) |
18.0 |
(10.8–25.2) |
18.1 |
(12.2–23.9) |
16.2 |
(5.4–27.0) |
| Underweight |
2.4 |
(2.2–2.6) |
0.3 |
(0.0–0.9) |
0.0 |
— |
0.0 |
— |
1.5 |
(0.0–4.4) |
| * BMI missing = 51, † BMI missing = 4, ‡ BMI missing = 8, ◊ BMI missing = 3 |
Discussion
Four out of five general practice patients with non-insulin-treated T2D self-monitored BG either daily or occasionally. Two-thirds self-monitored fasting BG and half self-monitored post-prandial BG, at least weekly. The mean patient HbA1c level was 7.1% (54.1 mmol/mol), and was significantly higher for those who self-monitored daily than for those who never self-tested. These results show that while SMBG is common among patients with non-insulin-treated T2D, no beneficial clinical outcome is discernible in terms of HbA1c levels.
The BMI levels were not different for patients who self-monitored compared with those who did not, and collectively the proportion of obese patients was nearly double that of all patients attending general practice.17 The IDF suggestion that self-monitoring may induce lifestyle changes and weight reduction3 is not supported by these findings; neither did we find evidence that clinicians are encouraging overweight/obese T2D patients to self-monitor their BG.
Results should be considered in light of the study’s limitations. Prevalence estimates are waiting-room estimates, not population estimates. Older patients with multiple morbidity are likely to attend more frequently and therefore more likely to be sampled in BEACH. The effect of self-monitoring over time could not be measured with cross-sectional point estimates – patients may have had better (or worse) HbA1c levels previously or may have been influenced by interventions other than self-monitoring. Time since diagnosis was not asked – it is possible that patients who self-monitor daily had significantly higher HbA1c levels than patients who never test, because they are newly diagnosed and not yet controlled. They may also include patients with more severe diabetes who struggle for control. However, the numbers who self-monitor far exceed the proportion who would have been recently diagnosed. In a previous sub-study, only 5.6% of T2D patients had been diagnosed in the previous year, and over 80% more than five years previously.19
The 79% who reported SMBG is consistent with another finding, that 83.7% of patients with T2D had their own monitoring device. For 44.7% of these patients, the device had been recommended by their GP, and for 35.3%, by a diabetes educator. Smaller proportions reported the devices being recommended by an endocrinologist (6.7%), or pharmacist (7.3%).20 Such recommendations encourage patients with non-insulin-treated T2D to self-monitor, despite the lack of evidence supporting clinical benefits21 or cost-effectiveness,9,22 and regardless of reported negative outcomes.5,9,10,22 Guidelines are ambiguous, advising clinicians that while there is no conclusive evidence of benefit, the current leaning is to recommend SMBG as there may be some benefit to some patients in some circumstances, with appropriate training and education.3,4,23
Implications for general practice
While the cross-sectional data have less strength than interventional data, these results concur with findings from the published randomised control trials (RCTs) reviewed for this work, and with the growing body of evidence that the practice of SMBG is of little benefit to most patients with non-insulin-treated T2D. They further inform the DUSC/PBAC review on the utilisation and patterns of SMBG, and the proportion of patients with T2D who may be affected by the outcome of the review.13
Ethics approval: The BEACH program and SAND sub-studies are approved by the Human Research Ethics Committee of the University of Sydney (Ethics protocol Ref. No. 11428).
Provenance and peer review: Not commissioned; externally peer reviewed.
Acknowledgements
We thank the GP participants for their generosity, all members of the BEACH research team and particularly A/Prof Helena Britt for reviewing this manuscript prior to submission.
In 2011–2012, the BEACH program was funded by the Australian Government Department of Health and Ageing, AstraZeneca (Australia), CSL Biotherapies Pty Ltd, GlaxoSmithKline Australia Pty Ltd, Merck, Sharp and Dohme (Australia) Pty Ltd, Novartis Pharmaceuticals Australia Pty Ltd, Pfizer Australia Pty Ltd, Sanofi-Aventis Australia Pty Ltd, and the Australian Government Department of Veterans’ Affairs. The SAND sub-study reported in this manuscript was undertaken in collaboration with Sanofi-Aventis Australia Pty Ltd.
The BEACH program is funded by a consortium of pharmaceutical manufacturers, Australian government agencies and government-funded quality of care agencies such as the National Prescribing Service. Each group provides funding through a research agreement with the University of Sydney, which provides complete research autonomy for the team conducting the BEACH program. Funders provide input into the research design development through an advisory board however the final decisions regarding research design, data collection and analysis, and reporting of findings remain with the principal investigators under the ethical supervision of the University.
Each funding organisation has the right to nominate two topics for the BEACH/SAND sub-studies. The organisation works with the BEACH team to formulate the research questions for the SAND questionnaire. The final decision on the acceptability of the research questions rests with the BEACH team. All SAND questions are subject to approval by the Human Research Ethics Committee of the University.
Once the SAND questions are approved, the processes of data collection, analysis and reporting are conducted exclusively by the BEACH research team, including the development of published abstracts of the research.
Any peer-reviewed papers developed from SAND sub-studies are authored or co-authored by the BEACH research team. The funding organisation supporting the study has no editorial control over any aspect of the paper including the presentation of results. No financial support for production of the paper is accepted from the funding organisations.