Patients born, raised or recently arrived from high TB-burden countries, and patients from some indigenous communities, are at much higher risk of contracting TB. After infection, TB may remain latent for many years, re-emerging with immunosuppression or advancing age. Testing for TB varies according to the stage of disease. Latent TB infection is diagnosed by detecting specific immunological responses to Mycobacterium tuberculosis (MTB) proteins using interferon gamma release assays or tuberculin skin testing. For active TB, chest X-ray and microbiological tests are required. This article outlines how each test can contribute to a diagnosis of TB, although a key diagnostic challenge is still to 'remember TB'.
What is tuberculosis?
Tuberculosis is caused by infection with strains of the mycobacteria complex, typically MTB. Other closely related species such as M. africanum (human) and M. bovis (bovine TB) are also responsible for infection, although these are uncommon in Australia. Other mycobacterial species may cause infection but are not contagious, require different treatment and should not be termed tuberculosis. Tuberculosis is a notifiable disease in Australia, by both laboratories and clinicians. Household contacts of a confirmed case of TB require assessment.
Following exposure and inhalation of airborne tubercle bacilli, cellular immune defences usually contain the infection so patients do not become acutely unwell. Occasionally (and particularly in children), infection disseminates resulting in TB meningitis or miliary TB. Primary infection may also manifest as pneumonia or pleural effusion. When infection is contained by the immune system it remains dormant, usually within the lung apices. This is termed 'latent tuberculosis infection' (LTBI).
Overall, a person with LTBI has a 10% risk of developing active TB during their lifetime, with the greatest risk being within 2 years of infection. Once reactivated, symptoms such as fever, night sweats, weight loss and cough develop, typically for 2–3 weeks or more.
Tuberculosis is classically a pulmonary disease, but 39% of Australian notifications in 2007 were extrapulmonary infections.1 Other sites of infection include lymph nodes, pleural space, bone and joints, genito-urinary and meninges.
When to consider TB
As presentations may be atypical, TB should always be considered, particularly in populations at high risk of TB exposure (Table 1) or of TB reactivation (Table 2). A flowchart detailing how to investigate when considering a diagnosis of TB is shown in Figure 1.
Table 1. Populations at increased risk of exposure to TB
- Elderly patients – the incidence was far higher in the mid-20th century, particularly postwar European migrants and refugees from the Vietnam war
- Aboriginal Australians
- Torres Strait Island residents – from exposure to Papua New Guinean residents (where the incidence of TB and MDR-TB is high)11 crossing the border
- Migrants or refugees from high TB-burden countries, particularly refugees from sub-Saharan Africa and students from India, China and other Asian countries
- Healthcare workers who have been working in high burden countries
|
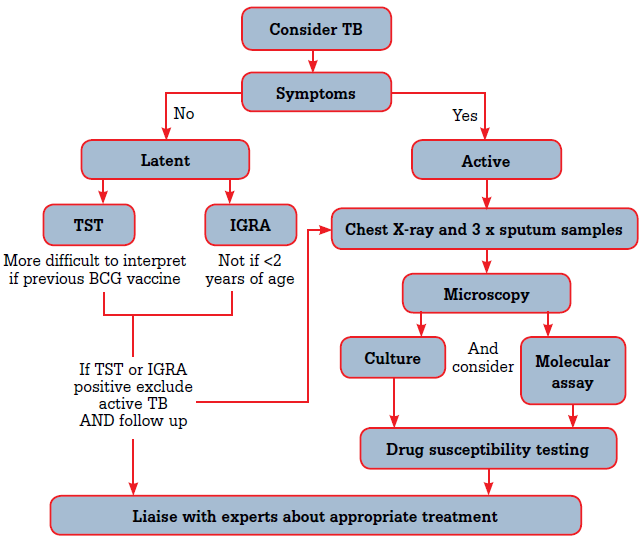
Figure 1. Investigations when considering a diagnosis of TB
Table 2. Risk factors for TB reactivation
- Recently acquired infection – particularly within the first 2 years
- Immunocompromised states including HIV infection (risk of TB reactivation is 10% per annum4 although this is substantially reduced with antiretroviral therapy12)
- Medicines related (eg. immune modulators, chemotherapy and post-transplantation)
|
Tests for suspected pulmonary TB
Patients with symptoms suggestive of active TB (such as night sweats, cough and fever) require chest X-ray and microbiological testing. These patients are potentially infectious so precautions should be used.
Chest X-ray
Both posterior-anterior (PA) and lateral films are important with clear notes to the radiologist. Typical changes include air space consolidation, cavitation and fibrous contraction in one or both upper lobes or superior parts of the lower lobes (Figure 2). In human immunodeficiency virus (HIV), atypical findings are more common, including [rarely] no changes despite active disease. It can be difficult to distinguish active from latent infection on chest X-ray so microbiology is also important.
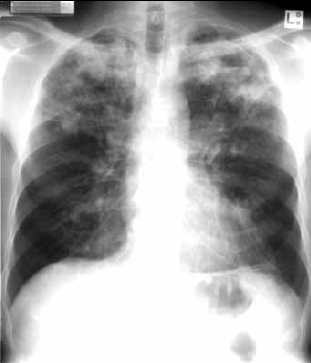
Figure 2. Chest X-ray of a man, 47 years of age, with typical pulmonary tuberculosis
Sputum testing
Three sputum specimens (preferably early morning) are indicated to maximise yield. In children, gastric aspiration is a useful surrogate. Nebulised hypertonic saline may induce sputum production and rarely bronchoscopy is used. Collection should occur away from other people, and into a jar with a threaded lid. Sputum should be delivered promptly to the laboratory but can be stored in the refrigerator for 1–2 days.
Microscopy
Acid-fast microscopy is rapid (within 24 hours, less than an hour if urgent) and inexpensive. Approximately 5000 bacilli per mL of sputum are required for a positive smear. Microscopy detects acid-fast bacilli in ~50% of pulmonary TB. The Ziehl-Neelsen stain is standard, and utilises the particular staining properties of the waxy coats of the mycobacterial cell walls. Culture or molecular assays are then required to confirm if acid-fast bacilli are MTB complex.
Culture
Culture is the most sensitive test. Automatic liquid cultures are preferred as they are more rapid and sensitive than solid culture mediums and can also test drug susceptibility.
Molecular assays
Molecular assays detect DNA specific for MTB complex. In smear-positive samples they are highly sensitive, but in smear-negative specimens, sensitivity drops to 50%. A new rapid polymerase chain reaction (PCR) test (Xpert® MTB/RIF assay, Cepheid, Sunnyvale CA.) that is not prone to contamination is advocated by some experts.2 Sensitivity is equivalent to solid culture and it simultaneously detects rifampicin resistance.
Drug susceptibility testing
The cultured isolate should be tested against first line treatment agents (isoniazid, rifampicin, ethambutol, pyrazinamide and streptomycin). Resistant strains are then tested against second line drugs. Multidrug resistant cases (MDR) are defined by resistance to both isoniazid and rifampicin. Rifampicin resistant strains are usually also isoniazid resistant. Drug resistant strains require longer treatment; for MDR-TB, World Health Organization guidelines recommend at least 20 months of therapy.3
Tests for suspected latent TB
Diagnosis of LTBI is dependent on tests of immune recognition of MTB antigens with tuberculin skin testing (TST) or interferon gamma release assays (IGRA). These tests become positive 4–6 weeks after infection.4 Presentations may include patients from high prevalence countries, contacts of an index case or healthcare workers presenting for TB screening.
Why diagnose latent TB?
Treating LTBI decreases the risk of progression to active TB by 60–90% and eliminates the potential for transmission. Preventive chemotherapy is typically with isoniazid alone (9 months on average) rather than requiring combination therapy. The risk of progression to disease is greatest within the first 2–3 years, and especially within 1 year.5
The tuberculin skin test
The TST – or Mantoux test – assesses inflammation in the dermis following intradermal injection of tuberculin protein. The test needs to be read 48–72 hours after administration. The diameter of induration gives a semiquantitative assessment of the likelihood of LTBI. False positives can result from previous bacilli Calmette-Guérin (BCG) vaccination and exposure to environmental Mycobacterium spp. General practitioners should liaise with their local laboratories about the availability of testing in their area.
Interferon gamma release assays
Interferon gamma release assays utilise the ability of human lymphocytes to survive for a short period in a test tube. If primed by previous TB infection, lymphocytes will produce detectable amounts of gamma interferon. There are two commercially available tests: the Quantiferon Gold In-Tube™ assay (Celestis Australia) and the T-spot™ test (Oxford Immunotech). Interferon gamma release assays are unaffected by previous BCG vaccination. The venous sample does not require special timing or preparation by the patient. A Medicare rebate is available only if the patient is immunosuppressed. General practitioners should liaise with their laboratory as the assay can cost the patient up to $100.
A positive IGRA suggests that the patient's immune system recognises TB antigens. This may be due to either current infection or a remote past infection. Interferon gamma release assays do not diagnose active TB – microbiological tests are needed. Similarly a negative test cannot exclude active TB because sensitivity is not 100%.6
Which test is best in LTBI?
Despite numerous trials and subsequent meta-analyses, how best to incorporate IGRAs into diagnostic algorithms remains somewhat controversial. Australia's National Tuberculosis Advisory Committee suggests TST remains the preferred method with IGRAs as a supplemental assay in subjects more than 2 years of age.7 International guidelines vary considerably.8 Tuberculin skin testing is preferred in young children, especially in those under 2 years of age. Interferon gamma release assays are easier to interpret in patients vaccinated with BCG and should perform better in immunosuppressed patients, but there is no evidence of this. The QFG-IT assay facilitates assessment of the immune system response. No detectable immune response (anergy) can cause a false negative TST test. Table 3 compares important features of the two main types of tests for detecting LTBI.
If a TST or IGRA are positive, then active disease must be excluded with chest X-ray and sputum for microscopy and culture. Any positive result needs to be discussed with a clinician experienced in TB management before commencing treatment.
Table 3. Comparison of important features of the two main types of tests for detecting LTBI
| | TSTs | IGRAs |
|---|
| Medicare Benefits Schedule |
$11.30 |
$35.15 (for immunosuppressed or immunocompromised patients only)* |
| Specificity – influenced by previous BCG vaccination |
Yes |
No |
| Specificity – influenced by previous infection by environmental mycobacteria |
Yes |
Less than with TST (but test antigens also found in M. marinum, M kansasii and M. szulgai) |
| Special precautions |
Store tuberculin re-agent at –8°C and protect from light |
Blood must be processed within 16 hours |
| Suitable in children <2 years of age |
Yes |
No |
| Hypersensitivity reactions |
Possible – anaphylaxis
rare |
No |
| Number of visits to complete test |
Two# |
One |
* Note: there may be additional costs even in immunocompromised patients – liaise with your laboratory
# Note: with trained healthworker – liaise with your pathology collection centre |
Other testing
Extrapulmonary TB is diagnosed by sending a sample in normal saline for microscopy and culture. Histopathology may identify typical changes (necrotising granulomas, caseation and acid-fast bacilli.) General practitioners are advised to liaise with their laboratory about the best sample handling if they have a suspected case.
Serological tests for TB are commonly performed in some overseas countries, but these are insensitive and nonspecific and not recommended in Australia.9
All patients with TB should be screened for HIV infection. Liver enzymes should be monitored as several treatment drugs may cause a chemical hepatitis. Hepatitis B and C appear to increase the risk of hepatotoxicity, hence check serology before treatment.10 Regular full blood examination is indicated to look for drug induced neutropenia or thrombocytopenia. Vitamin D deficiency may increase the risk of TB progression, although the value of assay and replacement is unclear.
Key questions in TB diagnosis
- Could this be TB? Consider TB in symptomatic patients, in patients from populations at high risk and in those with undifferentiated illness.
- Is this active or latent TB? Testing is different depending on the stage of disease.
- In latent TB – is TST or IGRA more accessible and appropriate in this patient?
- In active TB – after chest X-ray order sputum microscopy and culture. Should I request molecular testing to speed diagnosis and detect resistance?
Conflict of interest: none declared.
Acknowledgements
The contribution of Dr Andrew Burke in reviewing the manuscript is gratefully appreciated. Acknowledgement is also given to the Department of Medical Imaging, The Prince Charles Hospital, Brisbane, for use of the chest radiograph image.