Histological confirmation of a melanoma diagnosis
Even when a confident clinical diagnosis of melanoma is made, excision biopsy – rather than immediate wide excision – should be performed. This confirms the diagnosis and allows rational planning of definitive treatment – width and orientation of excision margins, and whether or not to recommend sentinel lymph node (SLN) biopsy. Immediate wide excision with margins based on a clinical estimate of tumour thickness may result in inadequate or excessive tumour clearance. It may also compromise subsequent management by making it impossible to perform accurate lymphatic mapping to identify draining lymph node fields and SLNs within those fields.
The Clinical practice guidelines for the management of cutaneous melanoma in Australia and New Zealand,3 endorsed by the Australian National Health and Medical Research Council, recommend excision biopsy with 2 mm margins whenever possible. Partial biopsies such as punch biopsies, incision biopsies and shave biopsies are frequently unsatisfactory and may result in misdiagnosis due to unrepresentative sampling. The great majority of legal cases brought against doctors for alleged mismanagement of patients with melanomas are associated with incomplete biopsies.4 Nevertheless, an incision, punch or shave biopsy from the most suspicious area of a large pigmented lesion may be appropriate when complete excision would be difficult.
Definitive treatment by wide excision
The width of excision required for a particular melanoma is based primarily on its Breslow thickness (Figure 1). The excision margins for melanomas of various thicknesses recommended in evidence based Australian and New Zealand guidelines are shown in Table 1. Moh's surgery is generally considered inappropriate for definitive treatment of a melanoma.5
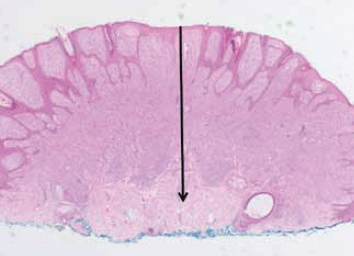
Figure 1. Primary melanoma on the arm of a woman aged 25 years. The arrow indicates the Breslow thickness. This is defined as the microscopically measured vertical depth of invasion of the tumour from the granular layer of the epidermis to its deepest part within the dermis or subcutaneous tissue
Table 1. Melanoma wide excision margins (after initial excision biopsy) recommended in the Clinical Practice Guidelines for the Management of Cutaneous Melanoma in Australia and New Zealand3
| Breslow thickness* | Surgical margin |
|---|
| Melanoma in situ |
5 mm |
| Melanoma <1.0 mm |
1 cm |
| Melanoma 1.0–4.0 mm |
1–2 cm* |
| Melanoma >4.0 mm |
2 cm |
| * For melanomas 2–4 mm thick, it may be desirable to take a 2 cm margin where possible |
When is assessment of regional lymph nodes indicated?
For in situ melanomas, there is a negligible risk of spread to regional lymph nodes. For invasive melanomas <0.75 mm in Breslow thickness, the risk of spread to regional lymph nodes is very low (<5%). Hence, wide local excision (with 5 mm) margins is all that is required.
For melanomas 0.75–1.0 mm thick, the likelihood of microscopic involvement of a SLN at presentation is 5–10%.6 The risk is greatest in the presence of histological features such as ulceration or an elevated tumour mitotic rate (>1/mm2).6 Sentinel lymph node biopsy may sometimes be considered in this subgroup.
For intermediate thickness melanomas (1.0–4.0 mm in Breslow thickness), the risk of metastatic spread to regional lymph nodes is 15–25%, rising with increasing tumour thickness.7 For thick melanomas (>4.0 mm in Breslow thickness), SLN positivity rates are 25–40%.8 The risk of systemic metastasis is also high, whereas for the intermediate thickness group, the risk of systemic metastasis is much lower. Therefore, patients with intermediate thickness melanomas are most likely to benefit from early intervention to remove microscopic disease in regional lymph nodes, identified by the SLN biopsy procedure.9,10 There is emerging evidence from a large, randomised multicentre clinical trial (the first Multicenter Selective Lymphadenectomy Trial, MSLT -I) that there is a substantial survival benefit for SLN-positive patients if they have an early complete lymph node dissection (CLND); 5 year survival was 72.3% after early CLND vs 52.4% after dissection when regional node metastasis became clinically apparent, p=0.004.11,12 The interim analysis did not reveal a statistically significant difference in overall melanoma specific survival for patients who had wide excision and SLN biopsy compared with those who had wide excision only. The final analysis of MSLT -I should be available in late 2012.
Sentinel lymph node biopsy currently provides the most accurate prognostic information available for melanomas of all thicknesses (Figure 2a, b). In MSLT -I, the 5 year survival for patients who were SLN negative was 90.2% whereas for those who were SLN positive it was 72.3%.11 This prognostic information is regarded as valuable by most patients and their doctors.
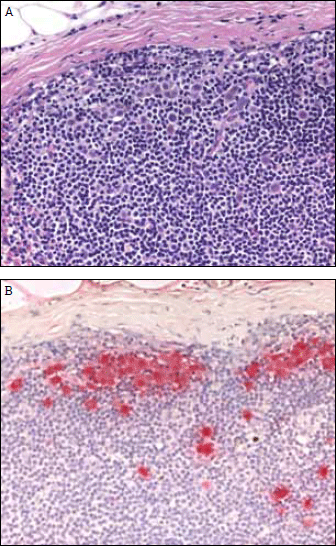
Figure 2. Melanoma metastasis in a sentinel lymph node. A) The large melanoma cells are present in a background of numerous small lymphocytes (haematoxylin and eosin stain) B) The melanoma cells are more easily identified with an S-100 protein immunohistochemical stain
The Australian and New Zealand guidelines recommend discussion of SLN biopsy with patients who have melanomas ≥1 mm in thickness. Although simple in concept, lymphatic mapping, SLN biopsy and histological SLN assessment are specialised techniques, best performed by individuals with appropriate training and experience. Lymphatic mapping may be inaccurate after wide excision, therefore consider direct referral of patients who might benefit from SLN biopsy to a melanoma centre or surgical oncologist.
Approximately 15–25% of patients with intermediate thickness melanomas are SLN positive. The current treatment recommendation in this circumstance is an immediate CLND.3 However, a second Multicenter Selective Lymphadenectomy Trial (MSLT -II) is currently randomising patients who are found to be SLN positive either to have standard therapy (immediate CLND) or to have monitoring of their remaining lymph nodes with high resolution ultrasound. Until the results of the MSLT -II are available, CLND remains the standard treatment for patients who are found to be SLN positive.
What staging investigations are required?
Staging tests, including blood tests, computed tomography (CT) scans and positron emission tomography (PET) scans are not recommended in patients who present with American Joint Committee on Cancer (AJCC) stage 1 or stage 2 disease (ie. with an invasive primary melanoma of any thickness and no clinical evidence of metastatic disease).13
Discussing a prognosis with the patient
The Melanoma Staging Committee of the AJCC has produced a free, web based prognostic calculator derived from analysis of a large dataset of patients with long term follow up (see Resources). For example, a patient aged 54 years with an 0.8 mm nonulcerated extremity melanoma has predicted 5 and 10 year survival rates of 98% and 96%, whereas a patient aged 60 years with a 7.0 mm ulcerated melanoma of the extremity has 5 and 10 year survival rates of 47% and 30%.13 Another free prognostic calculator is available as an iPhone app (see Resources).
As well as Breslow thickness of the primary tumour and SLN status, important negative prognostic factors are a high tumour mitotic rate and the presence of ulceration. Other important factors are the gender and age of the patient, with older men having a particularly poor outcome. Previously Clark level of invasion was used as an indicator of prognosis, but in the AJCC database, multivariate analysis showed that it was a much less important prognostic factor.14 In the most recent version of the AJCC melanoma staging system, T1b melanomas were redefined as tumours ≤1 mm in Breslow thickness with ulceration or a mitotic rate ≥1/mm2, regardless of Clark level.15
Management of stage 3 (metastatic) disease
The standard treatment recommendation for microscopic or clinically detectable metastatic melanoma in lymph nodes is regional lymph node dissection. Fine needle biopsy is preferred to confirm involvement, because recurrence risk is greatly increased if open biopsy is performed. Lymph node clearance is more extensive than in other cancer treatments. To minimise the morbidity of cervical lymph node dissection, a 'selective' dissection may be used, tailored to the site of the primary tumour. For axillary node involvement, a complete, three-level axillary lymph node clearance is required. For groin node involvement, a full subinguinal node clearance is required, with iliac and obturator node clearance if there are large or multiple nodal tumour deposits below the inguinal ligament. Staging with CT and PET scans is worthwhile in patients with stage 3 disease to determine the extent of metastatic spread. Magnetic resonance imaging scans of the brain are the most sensitive screen for brain metastases, which may not be detected on PET scans.
Management of intransit metastases
Up to 10% of melanoma patients develop recurrence between the primary tumour site and draining regional lymph nodes (intransit metastases).16,17 Various treatment modalities are available,18,19 preferably by a specialist melanoma treatment centre.
Management of systemic metastases
Isolated systemic metastases are best treated by surgical excision, and 5 year survival rates exceeding 40% have been reported following resection of one or more systemic metastases in up to three body cavities.20
Systemic therapies for systemic metastatic disease previously produced very low response rates. However, new drugs are providing genuine progress for patients with systemic melanoma metastases. Fifty percent of melanomas carry an activating mutation in the BRAF oncogene which drives proliferation and inhibits apoptosis. Two orally administered inhibitors, vemurafenib and dabrafenib, target this mutation. More than 50% of patients treated have objective remissions in all sites of disease, including the brain.21,22 The brain is a site of metastasis in >20% of metastatic melanoma patients at diagnosis and is a major cause of death. The BRAF inhibitors have few side effects, except cutaneous squamous cell carcinomas (SCCs). Resistance is problematic, although combinations with other agents are showing promise. They are not yet approved on the Pharmaceutical Benefits Scheme, but are available in clinical trials.
Another new approach, immunotherapy with ipilimumab, stimulates T-cells by inhibiting a regulatory cell surface molecule. This prolongs overall survival in patients with metastatic melanoma, with 20–30% of patients surviving 3 years.23 Autoimmune toxicity, such as colitis, is common but reversible with corticosteroids. Pharmaceutical Benefits Scheme approval is being sought, but ipilimumab is already available through major melanoma centres on a restricted basis as part of ongoing clinical trials.
Follow up
The purpose of follow up is to detect melanoma recurrences and new primary melanomas. For recurrence detection, there is little evidence to guide follow up intervals,24,25 but it seems logical to see patients at greatest risk of recurrence (ie. those with thicker tumours, ulceration and/or a high mitotic rate) more often than those with a statistically low risk of recurrence. Thus, a patient with a thin melanoma (&1 mm) probably only needs to be seen annually, whereas a patient with a thick melanoma (>4 mm) should probably be checked at least every 4–6 months for the first 2–3 years (when the risk of recurrence is greatest), and less frequently thereafter. Follow up designed to detect new primary melanomas will of course need to be more frequent in patients who are at particularly high risk (eg. when they have a strong family history, dysplastic naevus syndrome, or a history of one or more previous primary melanomas).
Key points
- Excision biopsy with 2 mm margins is appropriate whenever possible.
- Wide excision can then be based on the Breslow thickness.
- Tumors >1 mm thick may benefit from SLN biopsy.
- SLN biopsy provides accurate prognostic information.
- Staging investigations are not indicated unless metastasis is evident.
- Careful follow up is important to detect new melanomas and disease recurrence.
Resources
Conflict of interest: John thompson has received payment for consultancy from GlaxoSmithKline and Roche Australia. Richard Scolyer has received payment for consultancy, travel and accommodation from GlaxoSmithKline Australia Pty Ltd, Roche Australia, Abbott, the Taiwanese Dermatology Association and the British Melanoma Study Group. Richard Kefford has received payment of consultancy or travel from Roche Australia, GlaxoSmithKline, Novarits Pharmaceuticals Australia Pty Ltd, Bristol-Myers Squibb and Sanofi Pharmaceuticals.