While there is interest in iron reduction therapy for cancer risk reduction,3 improvement of insulin sensitivity in metabolic syndrome4 and management of fatty liver disease not responding to lifestyle changes,5 the Australian Red Cross Blood Service Therapeutic Venesection program is currently restricted to patients meeting the criteria listed in Table 1, and who also meet the general eligibility criteria for volunteer blood donation.
Table 1. Eligibility criteria for Australian Red Cross Blood Service Therapeutic Venesection program
- Evidence of hereditary haemochromatosis:
- C282Y homozygosity
- C282Y/H63D compound heterozygosity
- Clinical iron overload supported by FerriScan ® MRI or liver biopsy
- Polycythaemia rubra vera
- Porphyria cutanea tarda
|
Patients meeting therapeutic venesection criteria with contraindications to volunteer blood donation (eg. comorbid angina, hepatitis C, cerebrovascular disease) need to be referred elsewhere for therapeutic venesection. Options include private pathology providers, public hospitals, haematologists and some GPs.
In the absence of contraindications, patients with elevated SF who do not meet eligibility criteria for therapeutic venesection may become volunteer whole-blood donors every 12 weeks.
Potential harms of frequent venesection therapy for a person without true iron overload include development of iron deficiency anaemia, reinforcement of a suboptimal management strategy for a biochemical abnormality, perpetuation of the myth that a genetic condition affecting family members exists, and the general venesection risks of venous scarring, phlebitis and vasovagal episodes.
Iron metabolism
Approximately 75% of the body's 3–4 g total iron is found within haemoglobin in red blood cells, 10–20% is stored in the protein ferritin and the remainder is found in the iron transport protein transferrin, as well as in myoglobin, cytochromes and as unbound serum iron.6
Synthesised by the liver, the hormone hepcidin regulates total body iron levels by controlling intestinal iron absorption.7 Under the strict control of hepcidin, daily iron losses of 1–2 mg from sloughed mucosal, gastrointestinal and skin cells are accurately offset by daily absorption of 1–2 mg from dietary sources. Only 10% of daily dietary iron intake is absorbed.2
Iron overload
The human body lacks an iron excretion mechanism. Table 2 outlines circumstances in which iron overload can develop.
Table 2. Causes of iron overload
| Mechanism of iron overload | Example |
|---|
| Inappropriately increased intestinal iron absorption |
- Hereditary haemochromatosis
- HFE-haemochromatosis
- Type 1: HFE mutation (HFE gene)
- Non-HFE haemochromatosis (rare)
- type 2A: haemojuvelin mutation (HJV gene)
- type 2B: hepcidin mutation (HAMP gene)
- type 3: transferrin receptor 2 mutation (TfR2 gene)
- type 4: ferroportin mutation (FPN1 gene)
|
Transfusional iron overload
1 unit packed red cells ≈250 mg iron |
- Multiple transfusions to treat anaemia due to:
- red cell aplasia (congenital or acquired)
- haemoglobinopathies
- myelodysplastic syndrome, leukaemia
- cancer or chemotherapy for cancer
- severe haemorrhage in haemophilia/surgery/trauma
|
| Iron-loading anaemias |
- α-thalassaemia
- β-thalassaemia
- Chronic haemolytic anaemias
- Congenital sideroblastic anaemia
- Congenital dyserythropoietic anaemia
|
| Hepatocellular chronic liver disease |
- Alcoholic liver disease
- Hepatitis B or C
- Nonalcoholic steatohepatitis
|
| Excess parenteral iron |
|
Assessment of iron overload relies on surrogate markers, including serum tests (transferrin saturation, serum ferritin), noninvasive magnetic resonance imaging (MRI) scans for hepatic iron concentration (FerriScan®), liver biopsy and quantitative phlebotomy.2,6
Whole blood contains 250 mg iron per 500 mL.
In HH, total body iron stores can be calculated from the volume of blood removed during weekly venesections. Removal of 4 g or more of iron (16 weekly venesections) without developing iron deficiency anaemia indicates iron overload.6
Hereditary haemochromatosis
Hereditary haemochromatosis is an autosomal recessive condition of progressive iron overload, usually due to homozygosity for the C282Y mutation in the HFE gene. This mutation causes inappropriately increased intestinal iron absorption at a rate 2–3 times greater than normal.8 Similar to type 1 diabetes being a metabolic condition of glucose homeostasis due to insulin deficiency, HH is a metabolic condition of iron homeostasis due to hepcidin deficiency.9
Approximately 1 in 200 people of Caucasian race are homozygous for the C282Y mutation. This mutation has much higher penetrance than the H63D mutation. C282Y homozygotes are at highest risk of developing total body iron overload whereas C282Y/H63D compound heterozygotes have much lower risk.8,10 Even if H63D homozygotes develop elevated serum iron indices, they are unlikely to develop total body iron overload.10,11
C282Y homozygosity confers risk of the multi-organ consequences of iron overload, including liver fibrosis, liver cirrhosis, hepatocellular carcinoma, cardiac arrhythmias, cardiomyopathy, diabetes, arthropathy, hypogonadism and skin hyperpigmentation. Organ damage can be averted with early diagnosis and appropriate venesection therapy, but this is challenging due to the variable, subtle and nonspecific symptoms in early disease.
Whereas the HFE gene test indicates the risk of eventually developing iron overload, iron studies indicate if iron overload is currently present. The HFE gene test is performed once, whereas iron studies are performed every time an assessment of current iron overload is required (Table 3). A typical schedule of venesections for a patient with HH and iron overload is presented in Table 4.
Table 3. Advice based on HFE genotype and serum ferritin
| Genotype | Prevalence in Caucasian Australians11,12 | Advice if serum ferritin is normal | Advice if serum ferritin is elevated |
|---|
| High risk HFE genotypes |
|---|
Highest risk
C282Y homozygous |
1 in 188 |
- Increased risk of future iron overload
- Check iron studies every 1–5 years
- Family members need testing13
|
- Begin venesections – candidate for therapeutic venesection
- Family members need testing13
- SF >1000 μg/L: refer to gastroenterologist, haematologist or physician with an interest in iron overload
|
Lower risk
C282Y/H63D compound heterozygous |
1 in 46 |
| Low risk HFE genotypes |
|---|
| H63D homozygous |
1 in 49 |
- Check iron studies every 1–5 years
|
- Not a candidate for therapeutic venesection but can become a volunteer blood donor if no contraindications exist
- Look for another cause of elevated SF apart from HH, especially alcohol consumption, metabolic syndrome, obesity, liver disease and inflammation
- Consider non-HFE haemochromatosis
- Family members don't need testing13
- SF >1000 μg/L: refer to gastroenterologist, haematologist or physician with an interest in iron overload
|
C282Y carrier
H63D carrier
No mutations |
1 in 8
1 in 4
3 in 5 |
- No further follow up needed13
|
Table 4. Venesection schedule
| Iron unloading phase, target serum ferritin ~50 μg/L |
|---|
- Weekly venesection of ~7 mL/kg (maximum 550 mL) whole blood
- Ensure pre-venesection haemoglobin >120 g/L
- Monitor Hb and SF
- Hb: is it safe to remove more blood? Delay for 1 week if pre-venesection Hb <120 g/L
- SF: is it safe to remove more iron? Monitor SF every 4–6 venesections, more often as SF approaches
100 μg/L
- It may take many months or even years to unload excess iron
- Oral vitamin B12 and folate supplements support erythropoiesis during frequent venesections
|
| Lifelong maintenance phase, target SF ~50–100 μg/L |
|---|
- Venesections to maintain SF ~50–100 μg/L
- Highly variable between individuals, often in the range 2–6 venesections per year
- Monitor SF at least every 12 months
|
Iron studies
Accurate diagnosis of a patient's total body iron stores requires careful interpretation of iron studies (Table 5). Serum iron exhibits diurnal variation14 and the ideal specimen for iron studies is a fasting morning sample where oral iron supplementation has been withheld for at least 24 hours before testing.13
Table 5. Interpretation of iron studies
| Iron study test name | Explanation | Iron as an analogy to money | Abnormal values
(vary from laboratory-to-laboratory) |
|---|
| Suggestive of low iron stores | Suggestive of high iron stores |
|---|
| Serum iron |
Unbound serum iron |
'Loose change in your pocket' |
<10 μmol/L |
>30 μmol/L |
| Total iron binding capacity |
Ability to bind even more iron |
'Greediness for more money' |
>70 μmol/L |
<45 μmol/L |
| Transferrin saturation |
- Iron absorbed from duodenum bound to a transport protein
- One molecule of transferrin binds two atoms of iron
|
'Money kept in your wallet' |
<16% |
>45% |
| Serum ferritin |
- Iron within a storage protein
- One molecule of ferritin binds 4500 atoms of iron
|
'The savings you have in your bank' |
<30 μg/L |
- >200 μg/L pre-menopausal women
- >300 μg/L men and postmenopausal women
- >1000 μg/L refer to gastroenterologist, haematologist or physician with an interest in iron overload
|
The most useful tests in the evaluation of iron overload due to HH are transferrin saturation and serum ferritin.15 Transferrin saturation >45% is sensitive and fairly specific for diagnosing HH, with increasing specificity when the threshold is increased to >55%. Serum ferritin is most useful in monitoring venesection requirement and venesection response in patients already diagnosed with HH.
Serum ferritin
While low SF is a sensitive and specific indicator of low total body iron stores, elevated SF is sensitive but very nonspecific for iron overload. While a normal SF rules out iron overload, only 10% of cases of elevated SF are due to iron overload (Figure 1). Chronic alcohol consumption, metabolic syndrome, obesity, diabetes, malignancy, infection and inflammatory conditions explain 90% of causes of elevated SF.6,16
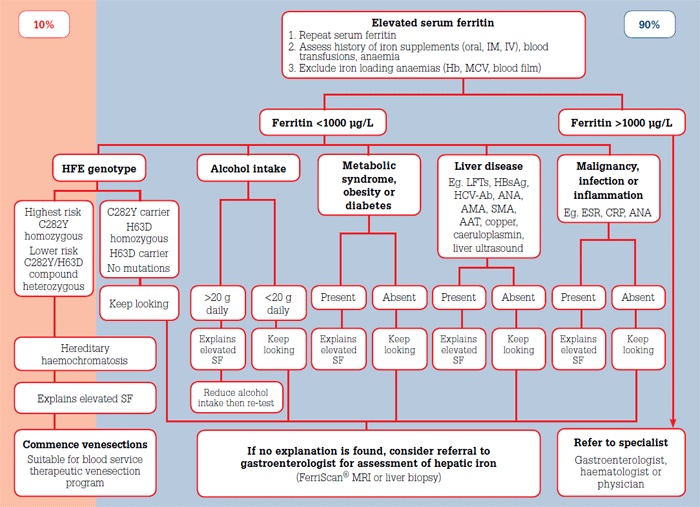
Figure 1. Algorithm for the investigation and management of elevated serum ferritin in general practice
Elevations of SF in the range 300–1000 μg/L are common, and often reflect the presence of the previously listed conditions. Mild elevations below 1000 μg/L are 'tolerable'6 and in the absence of HH, the risk of hepatic iron overload is exceedingly low.17
Australian studies have shown a link between alcohol consumption and elevated SF, with beer more so than spirits or wine causing increases in ferritin secretion by the liver.18 Chronic daily consumption of two or more standard drinks might explain elevated SF.19 Repeat SF testing after a period of alcohol abstinence can clarify the contribution of a patient's alcohol intake on their elevated SF.
There exists a well-established link between elevated SF, metabolic syndrome and fatty liver.20,21 With the Australian prevalence of metabolic syndrome being 1 in 3,22 the high pre-test probability of 'metabolic hyperferritinaemia' is important to consider when evaluating patients with elevated SF. Features which may discriminate elevated SF due to HH from metabolic hyperferritinaemia are listed in Table 6.
Table 6. Comparison between elevated serum ferritin in haemochromatosis and in metabolic syndrome
| Feature | Elevated serum ferritin due to hereditary haemochromatosis | Metabolic hyperferritinaemia due to metabolic syndrome/fatty liver/insulin resistance/diabetes/obesity |
|---|
| Genotype |
C282Y homozygous |
Not C282Y homozygous |
| Ancestry |
Usually Caucasian |
Variable |
| Transferrin saturation |
Usually >45% |
Usually normal (20–45%) |
| Serum ferritin |
Elevated |
Elevated |
| C-reactive protein |
Normal |
Normal |
Hepcidin levels
(not commercially available) |
Reduced hepcidin levels |
Normal or elevated hepcidin levels |
| Serum ferritin over time |
Progressively more elevated |
Fluctuations from one test to another |
| Total body iron levels |
Raised |
Normal |
| Response to weekly 500 mL venesections |
Patient tolerates >16 weekly venesections without becoming anaemic |
Patient becomes anaemic after <16 weekly venesections |
Hepatic iron concentration
(FerriScan ® MRI or liver biopsy ) |
Raised |
Normal |
| Pattern of iron deposition on liver biopsy |
Parenchymal deposition in hepatocytes |
Nonparenchymal deposition in sinusoidal and Kupffer cells |
| Management |
- Iron depletion
- venesections
- iron chelation therapy
|
- Lifestyle modifications
- weight control
- correction of insulin resistance
|
Liver disease is a cause of elevated SF. Injured hepatocytes leak ferritin into the serum, so in liver disease, SF can be considered as another type of liver function test (LFT), along with the transaminases (alanine transaminase [ALT], aspartate aminotransferase [AST]) and gamma-glutamyl transferase (GGT). Some causes of liver disease are associated with increased hepatic iron concentration (hepatitis B, hepatitis C, alcoholic liver disease, HH) so elevated SF with abnormal LFTs usually requires further investigation.23
Malignancy, infection and inflammatory conditions may all cause elevated SF. Normal screening tests for C-reative protein (CRP), erythrocyte sedimenation rate (ESR) and antinuclear antibody (ANA) can help exclude the presence of these conditions.
Specialist review is mandatory if SF exceeds 1000 μg/L due to the increased risk of fibrosis and cirrhosis above this threshold. However, in the absence of C282Y homozygosity, hepatic iron concentration is usually normal or only mildly elevated and fatty liver, hepatitis B, hepatitis C and alcoholic liver disease may be found.17,24
Key points
- Of all HFE genotypes, only C282Y homozygotes have a high risk of hepatic iron overload.
- Once HH has been excluded in a patient with elevated SF, assess for potential causes including chronic alcohol consumption, metabolic syndrome, obesity, diabetes, liver disease, malignancy, infection and inflammation.
- If SF >1000 μg/L, refer to a gastroenterologist, haematologist or physician with an interest in iron overload.
- If SF <1000 μg/L, address reversible causes and repeat iron studies.
- Encourage voluntary blood donation every 12 weeks.
Further information
Conflict of interest: none declared.
Acknowledgements
The authors thank Dr Barbara Bell, National Medical Services Manager, Australian Red Cross Blood Service for her assistance in providing referral data. Australian governments fully fund the Australian Red Cross Blood Service for the provision of blood products and services to the Australian community.