Case
A previously well man of Asian ethnicity, 63 years of age, presented with a four-month history of palpitations and typical features of hyperthyroidism, including unintentional weight loss, alopecia and diarrhoea. He was on treatment with lisinopril for hypertension. He was clinically thyrotoxic with a tachycardia of 160 beats per minute, but was haemodynamically stable. He had a moderate-size smooth goitre with a bruit that is consistent with Graves’ disease.
An electrocardiogram (ECG) showed atrial flutter and a rapid ventricular rate. Thyroid function tests confirmed thyrotoxicosis:
- free thyroxine (T4) 41.7 pmol/L (reference: 9.1–19.6)
- free triiodothyronine (T3) 23.9 pmol/L (reference: 2.4–5.9)
- thyroid stimulating hormone (TSH) <0.01 mU/L (reference: 0.30–5.00).
A Tc-99m pertechnetate thyroid scan showed diffuse increased tracer uptake consistent with Graves’ disease (Figure 1).
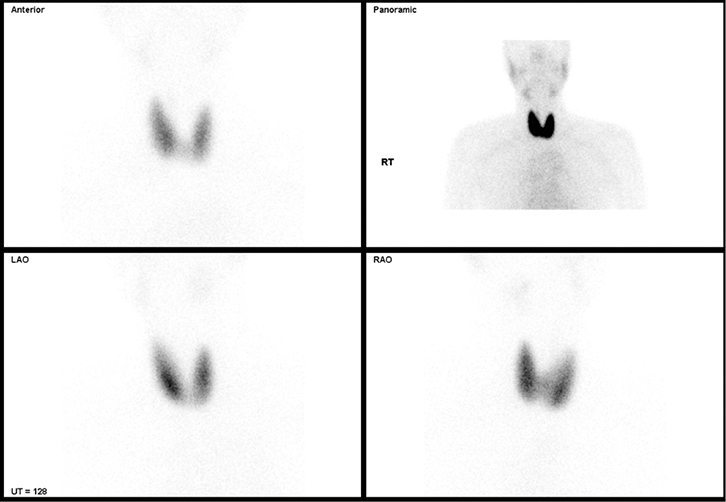 |
| Figure 1. Tc-99m pertechnetate thyroid scan revealing diffuse increased tracer uptake consistent with Graves’ disease |
The patient commenced carbimazole 20 mg bd and metoprolol 25 mg bd. Warfarin was initiated given the duration of symptoms, history of hypertension, and absence of contraindications to anticoagulation. The patient reverted to sinus rhythm within 48 hours. Thyroid function normalised within 40 days and the carbimazole dose was reduced. A 24-hour Holter monitor and transthoracic echocardiogram are planned at three months to help assess the patient’s ongoing need for anticoagulation.
Atrial fibrillation and thyroid disease
Hyperthyroidism is common, with an estimated population prevalence of 1.3% in the US.1 Incidence increases with age and is highest in white populations and iodine-deficient areas.1 Hyperthyroidism is associated with increased all-cause and cardiovascular mortality, and is linked to an increased incidence of embolic events and stroke, ischaemic heart disease, and congestive cardiac failure.2
Atrial fibrillation (AF) is a well-established complication of hyperthyroidism that may contribute to excess cardiovascular morbidity and mortality.3 We previously reported eight thromboembolic events in 70 patients with hyperthyroid AF and heart failure from a total of 381 patients with hyperthyroidism.4
In a population‑based study, 8.3% of hyperthyroid participants had newly diagnosed AF within 30 days of a hyperthyroidism diagnosis.5 A retrospective cross-sectional study of more than 23,000 hospitalised people aged 65–70 years, most of whom had underlying cardiovascular disease, reported a similar frequency of AF in subclinical hyperthyroidism (13%) and overt hyperthyroidism (14%), compared with 2% in euthyroid controls.6 In those younger than 60 years of age, with no additional risk factors for AF and a short duration of hyperthyroidism, reversion to sinus rhythm is expected within six weeks of return to a euthyroid state.3 However, if fibrillation persists after the patient has been euthyroid for four months, spontaneous reversion is unlikely to occur.7
Despite its prevalence, current evidence to guide decisions regarding anticoagulation in patients with AF and overt thyroid disease is lacking.8
Risk of thromboembolism in atrial fibrillation and thyroid disease
Excess thyroid hormone affects several coagulation and fibrinolytic parameters, and is not specific to the underlying disease process. In both subclinical and overt hyperthyroidism, a shift of haemostasis towards a hypercoagulable and hypofibrinolytic state, with a rise in factors VIII and IX, fibrinogen, von Willibrand factor, and plasminogen activator inhibitor-1, is observed.9
Unfortunately, there is limited data on the risk of embolic stroke in patients with hyperthyroidism and AF.9 Few randomised trials examining prevention of AF-related stroke have included patients with hyperthyroidism; in those that did, numbers were small.4 As such, there is insufficient evidence to determine the degree of increased stroke risk with hyperthyroidism and the role of prophylactic treatment. For this reason, present guidelines do not incorporate hyperthyroidism into stroke-risk evaluation.1,10 However, research has demonstrated age as an independent predictor of cerebral ischaemic events in thyrotoxic AF and, therefore, it should be integrated into the decision-making process on anticoagulation.1 Additional considerations include:
- other validated stroke and haemorrhage risk factors, such as the presence of cardiac failure, hypertension, diabetes, prior stroke and vascular disease, and gender and age, which contribute to the CHA2DS2VASc score11
- abnormal renal and liver function, bleeding history, labile international normalised ratios (INRs), alcohol and some other drugs and being elderly, which contribute to the HAS‑BLED score.12
It is important to recognise that the risk of AF-related thromboembolism from hyperthyroidism is not included in these scores; exclusive use of these scoring systems alone will underestimate the risk in hyperthyroidism. Just because there are insufficient data to quantify this risk does not mean that additional risk does not exist.
Safety of oral anticoagulants in atrial fibrillation and thyroid disease
Recent introduction of the novel oral anticoagulants (NOACs) has been welcomed by many for their fixed dosing regimen, lack of required monitoring, fast onset and offset of action, and fewer drug and food interactions. Two different classes of NOACs directly targeting factor Xa and thrombin are currently available in Australia for patients with non-valvular AF (Figure 2). However, the lack of clinically validated tests to monitor their anticoagulation effects may be detrimental in cases of altered pharmacodynamics such as hyperthyroidism. In patients with hyperthyroidism the turnover of vitamin K–dependent clotting factors is increased, which has been shown to enhance patient sensitivity to warfarin.13
There is no current literature on the effectiveness of NOACs in hyperthyroidism; however, given that their pharmacological effects are directly dependent on vitamin K–related clotting factors, it is likely that a similar increase in sensitivity will be observed. Furthermore, although an antibody fragment against dabigatran (idarucizumab) has been developed, as yet there are no reversal agents available in Australia for any NOACs.14 On the other hand, warfarin can be reversed relatively easily and at minimal expense. Therefore, warfarin may be considered the safest option because of its ease of monitoring and dose adjustment, our understanding of its pharmacodynamics in thyroid disorders, and the availability of a simple reversal agent.
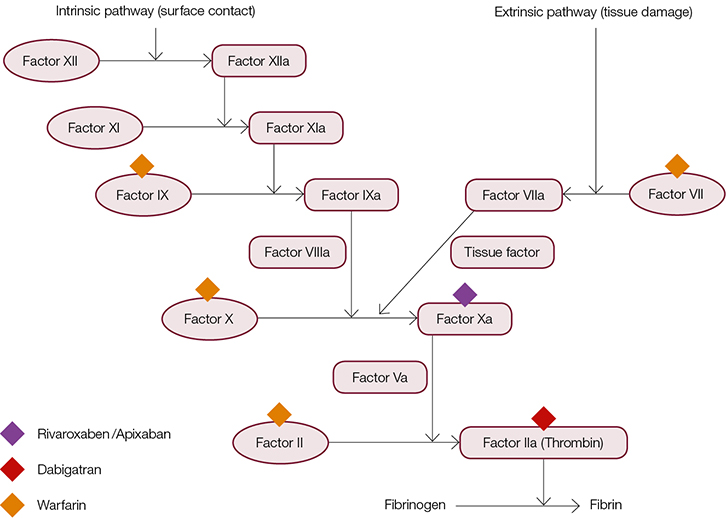 |
| Figure 2. Oral anticoagulants available in Australia and coagulation factor targets |
Conclusion
This review highlights the importance of recognising hyperthyroidism as a pro-coagulant state. While the in vitro effects on the coagulation system are well described, there is a lack of published literature to demonstrate hyperthyroidism as an independent risk factor for thromboembolic disease. Nevertheless, it is important that clinicians consider hyperthyroidism in addition to other documented risk factors when determining the appropriateness of anticoagulation for the prevention of thromboembolism in AF.
Ultimately, a final decision regarding the use of anticoagulation must be tailored to the individual patient and based on expected benefit versus harm. In light of the well-documented effects of overt and subclinical thyroid disease on haemostasis, and reports of significant effects on anticoagulant pharmacodynamics, if anticoagulation is deemed appropriate, we recommend the use of an easily measurable, titratable and reversible anticoagulant such as warfarin. Given the ongoing controversy regarding the risk of thromboembolic events in patients with AF and hyperthyroidism, it is important that clinicians regularly review a patient’s thyroid status and consider cessation of anticoagulation if euthyroidism is achieved.
Further research should be directed at clarifying the risk of thromboembolic disease in hyperthyroidism-induced AF, effectiveness and safety of available NOACs in thyroid disease, and duration of anticoagulation required once euthyroidism is achieved.
Authors
James M Polmear BPharm (Hons), MClinPharm, Clinical Pharmacist, Alfred Health, Melbourne, and Associate Director of Clinical Pharmacy, Barwon Health, VIC. j.polmear@alfred.org.au
Matthew J L Hare MBBS (Hons), BMedSc (Hons), Basic Physician Trainee, Department of Endocrinology and Diabetes, Alfred Health, Melbourne, VIC
Sarah R Catford BBioSc, MBBS (Hons), Advanced Endocrinology Trainee, Department of Endocrinology and Diabetes, Alfred Health, Melbourne, VIC
Duncan J Topliss MBBS (Hons), MD, FRACP, FACE, Director of Endocrinology and Diabetes, Department of Endocrinology and Diabetes, Alfred Health, Melbourne, VIC
Michael J Dooley BPharm, Grad Dip Hosp, Director of Pharmacy, Alfred Health, and Professor of Clinical Pharmacy, Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, Parkville, VIC
Competing interests: None
Provenance and peer review: Not commissioned, externally peer reviewed.