Ultraviolet (UV) radiation is produced by the sun and is a recognised causative factor in photo-ageing, and the development of melanoma and non-melanoma skin cancer.1 Since the early 1900s, sunscreens have been developed to try to block the harmful effects of UV radiation and protect human skin.2 Modern sunscreens contain a mixture of organic and inorganic ingredients that filter UV radiation. This review will solely examine concerns regarding the inorganic filters zinc (ZnO) and titanium (TiO2) as nano sized particles.
Brief overview of UV radiation
The sun produces UV radiation and the spectrum of UV radiation is subdivided into wavelengths (Figure 1). Three major subtypes of ultraviolet radiation exist:
- UVA: 320–400 nm
- UVB: 290–320 nm
- UVC: 260–290 nm.
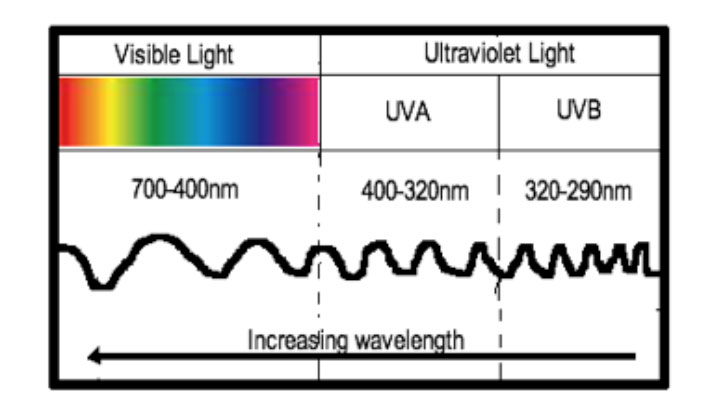 |
| Figure 1. Diagram depicting ultraviolet spectrum |
Most UVC is filtered by the Earth’s atmosphere; hence, it is not a causative factor in skin cancer.3 UVB is primarily responsible for causing sunburn and DNA damage, leading to the development of non-melanoma skin cancers. UVB was the primary target of early sunscreens. UVA is responsible for causing photo-ageing, photo-immunosuppression and photo-carcinogenesis.3 Modern ‘broad-spectrum’ sunscreens were developed to protect against UVA and UVB, and contain various ingredients to achieve this purpose.3
Sunscreen characteristics
Filters in sunscreens may be classified as organic or inorganic and predominately block either UVA or UVB (Figure 2). Organic ‘chemical’ filters include octinoxate (UVB), salicylates (UVB) and benzophenones (UVA). UV radiation is absorbed by the molecules in the filter, which then releases the energy via several mechanisms including fluorescence, photoreactions or redistribution of the energy within the molecule.3
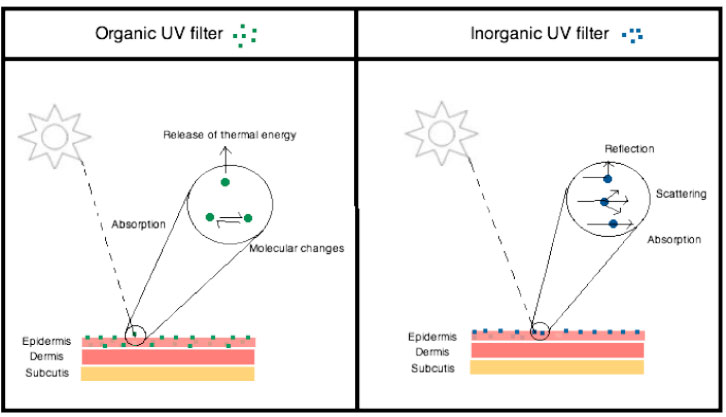 |
| Figure 2. Method of action of organic and inorganic UV filters |
The inorganic ‘physical’ filters, ZnO and TiO2, work by reflecting or scattering UV radiation. The efficacy of sunscreen is measured using sun protection factor (SPF). SPF measurement depends on the minimal erythema dose (MED), defined as the smallest UV dose that produces visible erythema of the skin at 16–24 hours after UV exposure.2
An ideal sunscreen includes ingredients that provide broad-spectrum protection against UVA and UVB, are resistant to water and sweat, and have a high SPF, ease of application, acceptable cosmetic appearance and inherent low risk of contact allergy.3
Concerns regarding nanoparticles
The inorganic filters ZnO and TiO2 are able to protect against UVA and UVB. As micronised particles in sunscreen, they have several unfavourable characteristics, including difficulty in application, leaving white residue on the skin, possibly staining clothing and being comedogenic. This led to the development of smaller nanoparticles, the use of which vastly improves cosmesis and ease of application of sunscreens while maintaining photo-protective qualities.3
Nanoparticles are defined as having dimensions in the order of 1–100 nm.4 Concerns relating to the use of nanoparticles are twofold. The first concern is the development of reactive oxygen species (ROS) from metal oxide nanoparticles on exposure to UV radiation. Some studies have shown these ROS to be potentially cytotoxic and carcinogenic.5 Additionally, there is conflicting evidence as to whether nanoparticles are small enough to penetrate the epidermis and be absorbed into the human blood stream, causing toxicity with long-term use.2
Recent research findings
Manufacturers have developed methods of coating ZnO and TiO2 nanoparticles to prevent ROS formation and reduce potential cytotoxicity by preventing adherence to human cells.2 Additionally, human skin has inbuilt barriers to protect against ROS.2 There is conflicting evidence for absorption through the skin. Jansen et al reported that nanoparticles are confined to the stratum corneum following numerous in vivo and in vitro studies, in animal and human skin, including damaged skin.2 Hence, with regular shedding of the stratum corneum, accumulation of nanoparticles does not occur.
The Therapeutic Goods Administration’s (TGA’s) Literature review on the safety of titanium dioxide and zinc oxide nanoparticles in sunscreens6 and the European Commission’s Scientific Committee on Emerging and Newly Identified Health Risks’ (SCENIHR’s) Risk assessment of products of nanotechnologies7 concluded that the evidence at the time of publication did not suggest that TiO2 and ZnO nanoparticles reached the general circulation. The TGA’s publication concluded that current evidence suggests these particles remain within the non-viable, keratinised cell layer of the stratum corneum and that short-term, in vivo studies did not demonstrate harm to human cells.6
These findings are in direct contrast to a previous study published in 2010 by Gulson et al, which found increased amounts of zinc in the blood and urine of human trial participants after a five-day application of sunscreen containing ZnO nanoparticles.8 Of concern, the concentration excreted peaked at nine days post-application, for reasons unable to be explained by the researchers.8
Additionally, an Australian study published in 2013 by James et al substantiated findings from several other studies published between 2006 and 2010, which showed that a small amount of Zn (either as nanoparticles or free ions) is able to penetrate healthy human skin and enter the circulation, stimulating an immune response.9 The authors concluded that macrophages play a dominant role in protecting the body against the cytotoxic effects of ZnO nanoparticles in in vitro studies.9 Further research into this area to determine the long-term clinical significance is required.
Future outlooks
The ability of nanoparticles to be absorbed in significant quantities needs to be further clarified. Additionally, the implications for long-term use, or use in damaged skin, must be established. Several studies have raised concerns regarding the potential photo-carcinogenicity of nanoparticles in sunscreens.5 To date, however, there has been inadequate evidence in human models.
It should be noted that the longest-running clinical trial assessing the effect of sunscreen on reducing skin cancer risk used organic sunscreens, not nanoparticle-containing sunscreens.10 Therefore, a randomised-controlled trial examining the effectiveness of nanoparticle containing sunscreens should be conducted to provide comparative information.
Currently, the SCENIHR states that topical use of nanoparticle-containing sunscreens does not pose a threat to human health, but does advise against use in areas with impaired barrier function, until more data in this area are available.2 Locally, the TGA’s 2013 report acknowledges that current evidence suggests nanoparticles remain largely in the stratum corneum; hence they do not cause harm to viable skin cells.6
Clearly, while conflict remains with regards to the absorption of nanoparticles and potential for cytotoxicity or photo-carcinogenesis with prolonged use, further research into this area needs to occur to determine the long-term health implications.
Implications for general practice
Given the known risks associated with UV radiation exposure, patients should be encouraged to continue with sun-safety behaviours, including the use of protective clothing, avoidance of exposure during peak UV radiation hours, and regular application of sunscreen. To date, the benefits of sunscreens in terms of reducing skin cancer risk far outweigh the potential risks of long-term use.
Unfortunately, manufacturers do not currently have an obligation to label particle size on sunscreens, making it difficult for consumers to identify sunscreens that do contain nanoparticles. The only method of reliably avoiding nanoparticle-containing sunscreens would be to choose a sunscreen containing only organic ‘chemical’ filters.
The Australasian College of Dermatologists provides clear information regarding the benefits of sunscreens and solar protection on its website. With specific reference to nanoparticles in sunscreens, it states ‘despite multiple publications about nanoparticles in magazines and newspapers, no adverse effect of nanoparticles in sunscreens have been demonstrated to date’.11 Similarly, The Royal Australian College of General Practitioners’ (RACGP’s) clinical guideline titled Sunscreen: Sun cancer prevention agrees with this sentiment.12
Cancer Council Australia has several helpful online resources regarding sun safety and, specifically, nanoparticles in sunscreens.13 Additionally, Choice provides a comprehensive article explaining nanoparticles in sunscreens14 along with a Guide to buying and using sunscreen,15 which may assist GPs in discussing these issues with patients.
Author
Penelope C McSweeney MBBS (Hons), FRACGP, MMed (Skin Cancer), DCH, DRANZCOG, Candidate, Master of Medicine (Skin Cancer), University of Queensland; General Practitioner, Redlands Clinic, Cleveland, QLD. penelopemcsweeney@gmail.com
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.