Gastrointestinal (GI) disease is common in Australia. Inflammatory bowel disease (IBD) is increasing, with an incidence of 23.7 per 100,000 individuals in Australia.1 Cancer surveillance programs have also emerged. The National Bowel Cancer Screening Program predicts that one in 23 Australians will develop colorectal cancer in their lifetime. The aim of the program is to continue to reduce deaths from bowel cancer through early detection of the disease with faecal occult blood tests (FOBTs).2–4 Patients who have had IBD for more than 30 years have a one in five chance of developing colorectal cancer (CRC).5 Microscopic colitis causes 10% of cases of chronic diarrhoea.6 A diagnosis of lower GI disease and cancer surveillance programs often requires colonoscopy and biopsy. Accurate clinicopathological assessment is important for the diagnosis and management of these diseases. In conveying a biopsy report to the referring doctor, the anatomical pathologist should ensure that the diagnosis and its significance are laid out in terms that are easily understood, and that lines of communication are open should there be any confusion. This article outlines indications for endoscopy, and histopathological findings with implications for common lower GI disorders, similarly to our previous paper on upper GI disorders.7
When to refer for colonoscopy
The American Society for Gastrointestinal Endoscopy suggests four main indications for GI endoscopy:8
- if the endoscopy result may change management
- if an empirical trial of therapy for benign digestive disease is unsuccessful.
- as an alternative to an initial imaging study
- if a therapeutic procedure is a primary consideration.
There are also screening or surveillance indications (eg IBD and CRC). ‘Alarm symptoms’ are another indication for colonoscopy in order to exclude organic disease. These symptoms include rectal bleeding, weight loss, iron deficiency anaemia, nocturnal symptoms, and a family history of selected organic diseases including CRC, IBD and coeliac disease.9,10 Alarm symptoms have been shown to have a low sensitivity and predictive value for malignancy, but these patients should still be referred to a gastroenterologist for assessment.9,10
There are a number of reasons to take biopsies of tissue during colonoscopy. These include:
- removal of polyps or detection of a colonic lesion (eg diagnosis of dysplasia or malignancy)
- macroscopic inflammation (eg IBD or other causes of colitis)
- surveillance of established diagnoses (eg IBD or previous malignancy).
If a patient presents with a history of diarrhoea, even if the colon looks normal, random biopsies (usually two from each colonic segment) are often taken to facilitate the diagnosis of microscopic colitis. The endoscopist should ensure that a relevant history and clinical question is included with the pathology referral. Ideally, the histology results will be ready within two to three days following colonoscopy.
Report and follow-up
Ideally, the endoscopist should make the general practitioner (GP) aware of the macroscopic findings along with any immediate recommendations. The anatomical pathologist should ensure that the histology report is laid out in terms that are easily understood, and copies should go to the proceduralist and GP. There should be clear lines of communication open should the GP have any inquiries about the results.
Interpreting the colonic biopsy report
Inflammatory bowel disease – Ulcerative colitis and Crohn’s disease
The diagnosis of IBD and classification to ulcerative colitis or Crohn’s disease relies on a combination of clinical, endoscopic and pathological features. Duration of symptoms and treatment can alter the histological findings, and endoscopy is often more reliable than histology for assessing the extent and activity of disease.11 Features of IBD include architectural, inflammatory and epithelial abnormalities. Histology features favouring the two diseases are well described in two reviews.11,12 If the histological features do not give a clear indication of subtype, a diagnosis of IBD unclassified (IBD-U) may be given until the diagnosis is established on clinical grounds,11,12 or conditions that mimic IBD (eg acute self-limiting colitis or infective-type colitis) are excluded. The report should also include an assessment of dysplasia, as there is an increased risk for the development of CRC in IBD.13 Studies examining this risk estimated this as 2%, 8% and 18% at 10, 20 and 30 years respectively after a diagnosis of ulcerative colitis.5 Meta-analyses examining the risk of CRC in Crohn’s disease state a relative risk of 2.5–4.5.5 Factors influencing the risk of developing CRC in IBD include:5,14
- duration and extent of disease
- previous history of dysplasia
- co-diagnosis of primary sclerosing cholangitis (PSC).
Stricturing disease in ulcerative colitis is also a risk factor for CRC.5
The National Health and Medical Research Council (NHMRC) has published guidelines on surveillance colonoscopy in IBD,14 but as a general guide, surveillance should begin after eight years of disease activity, or earlier if there is a strong family history for CRC.15 If PSC develops, surveillance colonoscopies should begin at this stage.15 Colonoscopies should be performed yearly if there are high-risk features (eg active ulcerative colitis, PSC, family history, stricturing disease or inflammatory polyps, or previous dysplasia), or three-yearly in quiescent disease without high-risk features.15 Patients with disease limited to the proctosigmoid (not including complicated anorectal disease in Crohn’s disease)15 may undergo age-specific CRC screening, as influenced by risk factors (see discussion on CRC below).5 The presence of high-grade dysplasia is an indication for colectomy. Low-grade dysplasia, especially if not associated with a raised lesion or considered endoscopically unresectable, may also be an indication for colectomy.5
Microscopic colitis
Microscopic colitis is a specific colonic pathology associated with symptoms of watery diarrhoea, when endoscopic findings are normal.16 There are two subtypes:17
- lymphocytic with chronic inflammation in the lamina propria and increased intraepithelial lymphocytes (≥15/100 epithelial cells)
- collagenous with a subepithelial band of collagen.
Microscopic colitis has an increasing prevalence and is common in elderly patients. Lymphocytic colitis is associated with autoimmune disorders and the diagnosis should also prompt investigation for coeliac disease. Drugs, particularly non-steroidal anti-inflammatory drugs (NSAIDs), can also induce microscopic colitis.17 Treatment options include loperamide, cholestyramine and steroids (budesonide has a better evidence base than prednisone; Case 1).18,19
Colonic polyps
There are two main types of colorectal polyps: adenomas and serrated polyps. Adenomas are precursor lesions to invasive adenocarcinoma, with increased risk of progression to malignancy if the lesion is large (>10 mm), has high-grade dysplasia or is villous in nature.20,21 Adenomas are classified as having villous, tubular or tubulovillous architecture, and are always composed of dysplastic epithelium. The more common tubular adenomas are characterised by elongated crypts lined by pleomorphic cells with pseudostratified, hyperchromatic, pencillate nuclei, occasional mitoses, and decreased cytoplasmic mucin. These same dysplastic cells line slender, finger-like projections extending above the crypts in villous adenomas, which have >75% villous architecture; tubular adenomas have <25% villous architecture; tubulovillous adenomas fall in between.22 The dysplasia is assessed as low grade or high grade. High-grade lesions will always be carefully assessed for evidence of early invasion.
Serrated polyps encompass hyperplastic polyps, sessile serrated adenomas or polyps (SSA/P), and traditional serrated adenomas (TSA).23 These lesions are characterised by the saw-toothed architecture of the crypt epithelium. Hyperplastic polyps are typically smaller, found in the left side of the colon with no dysplasia.23 SSA/P are flat lesions and distinguished from hyperplastic polyps by their distorted, dilated or laterally branched crypts, often with inverted epithelial maturation and surface mitoses.24 SSA/P have a tendency to be large, multiple and right-sided. Dysplasia can be present and there is clear evidence of progression to carcinoma.25 TSA are rare, located primarily in the left side of the colon and rectum, can be pedunculated or flat and are commonly dysplastic (Case 2).23
Other polyps seen in the lower GI tract include Peutz-Jaeger, inflammatory and juvenile polyps. Peutz–Jaeger-type polyps are hamartomatous polyps with broad, aborising bands of muscularis mucosa smooth muscle fibres. These are commonly seen as part of Peutz-Jaeger syndrome and should prompt assessment for this condition. Inflammatory polyps can be secondary to inflammatory disorders of the intestine or mucosal prolapse. Juvenile polyps are usually solitary rectal polyps, composed of ulcerated granulation tissue overlying dilated glands.22 Guidelines for polyp surveillance are shown in Table 1.5,14,26
Table 1. Colonic polyp surveillance4,14,26
|
| Risk category | Surveillance interval | Histology or endoscopic finding |
|---|
| Very low |
|
- No adenoma or rectosigmoid hyperplastic polyps
|
| Low |
- Five yearly
- If the first surveillance examination is negative, can resume ‘average risk’ surveillance (consider 10-yearly colonoscopy or faecal occult blood test every second year)
|
- One to two small tubular adenoma(s) (<10 mm)
|
| Intermediate |
- Three yearly
- Can consider lengthening the interval between surveillance colonoscopies if none of the features listed to the right are found at repeat study
|
- More than three adenoma (regardless of size)
- One adenoma if ≥10 mm
- Villous/tubulovillous adenoma
- Adenoma with high-grade dysplasia and complete excision*
|
| High risk |
- Yearly
- <Yearly
- Three to six monthly
- Then repeat at 12 months. If removal complete, surveillance should occur as outlined in this table
|
- Less than five adenoma
- Any polyposis syndrome
- ≥10 adenoma
- Piecemeal removal of large or sessile adenomas
|
| *This is the official recommendation supported by a number of organisations. However, from clinical experience, many gastroenterologists will repeat colonoscopy within 12 months of a finding of completely excised high-grade dysplasia.
At present, the follow-up for serrated adenomas is as for standard adenomas, as per the NHMRC guidelines. However, the American Gastroenterological Association recommends a three-year surveillance period for SSA/P if >10 mm or if dysplasia present, or if it is a TSA.
Note that there are separate recommendations for surveillance colonoscopy in IBD. This guideline can be accessed through the NHMRC.14
Surveillance after the age of 75 years depends on risk and comorbidities.
|
Colon cancer
Adenocarcinoma is the most common type of CRC. There are several recognised subtypes, including signet ring cell, mucinous, serrated, medullary, colloid, cribriform and micropapillary, with some variation in prognosis.27 Adenocarcinoma is classified as low grade or high grade, depending on the degree of gland formation (sometimes described as well-, moderately or poorly differentiated). However, this is difficult to quantify from the limited material provided by biopsy, and grading may be deferred until the tumour is resected. The carcinoma may be visualised as an obviously malignant mass at the time of colonoscopy or may arise within a dysplastic polyp. In the latter instance, the extent of submucosal invasion within the polyp will be reported, with deeper levels conferring an increased risk of residual disease (Case 3).28
Drugs and the GI tract
Various medications can cause histological damage in the GI tract, particularly NSAIDs, and the effects of common medications in the GI tract are shown in Table 2.29–33
Table 2. Drugs and the GI tract
|
| Drug | Biopsy findings |
|---|
| Non-steroidal anti-inflammatory drugs Corticosteroids |
Acute inflammation, reactive changes, nonspecific erosions and ulceration32,33 |
| Proton-pump inhibitors |
Microscopic colitis32 |
| Olmesartan |
Collagenous colitis32 |
Iron tablets, anthranoid laxatives
(including senna), anti-hypertensive agents |
Pseudomelanosis enteri/coli (brown pigment deposition)31,32 |
| Exogenous hormones, diuretics, sumatriptan, some herbal and weight loss products |
Ischaemic colitis30,32 |
| Ipilimumab |
Mimics IBD33 |
| Mycophenolate mofetil |
Range of injury from mild to severe including vascular congestion, erosions, ulcers, crypt abnormalities and inflammation29 |
| Chemotherapeutic agents |
Neutropaenic colitis, commonly with bacterial and fungal overgrowth32 |
Summary
An abundance of different pathologies with varied clinical manifestations occur in the lower GI tract and can be asymptomatic or cause marked morbidity and mortality. Accurate diagnosis is important so that treatment advice can be provided in a timely manner. Communication between the clinician and pathologist is paramount, particularly if there is doubt as to diagnosis.
Case 1
Mrs MC, aged 61 years, was referred to the gastroenterology clinic with chronic diarrhoea associated with faecal urge incontinence. She underwent colonoscopy, which was macroscopically normal. Random biopsies revealed collagenous colitis, showing a thickened subepithelial collagen plate with trapped superficial capillaries and an increased number of lymphocytes within the surface epithelium (greater than 15 lymphocytes per 100 epithelial cells; Figure 1). Mrs MC was asked to stop smoking, cease her proton pump inhibitor and avoid anti-inflammatory drugs. Initially, she was treated with loperamide and cholestyramine. Unfortunately, these interventions did not lead to symptom improvement. Next, she was treated with 50 mg prednisone (because it was difficult to access budesonide in the community), with excellent symptom improvement. She was weaned off prednisone over the subsequent 12 weeks.
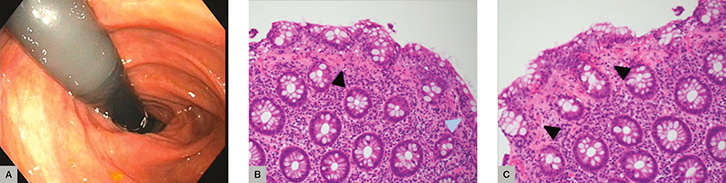 |
Figure 1. Case 1
(A) Normal endoscopy (B) Thickened subepithelial collagen plate with trapped superficial capillaries (black arrow) and increased number of lymphocytes within the surface epithelium, greater than 15 lymphocytes/100 epithelial cells (blue arrow) (C) Thickened subepithelial collagen plate (black arrow) |
Case 2
Ms SP, aged 64 years, was referred for a colonoscopy following a positive FOBT. A sessile polyp was resected from the ascending colon. Histopathology showed a polyp with a serrated lumen extending to the crypts with dilated, ‘boot-shaped’ distortion of the basal architecture, surface mitoses and focal low-grade dysplasia (Figure 2). A diagnosis of sessile serrated adenoma/polyp with low-grade dysplasia was made. Sessile serrated adenomas/polyps are predominantly right sided, display distinct crypt architectural changes and can have absent, low-grade or high-grade dysplasia. Regardless of the presence of dysplasia, recent studies have determined that SSA/P represent an important, alternative carcinogenesis pathway to conventional, non-serrated adenomas.25 As such, they require follow up. Ms SP falls into the low-risk category and will require five-yearly surveillance.
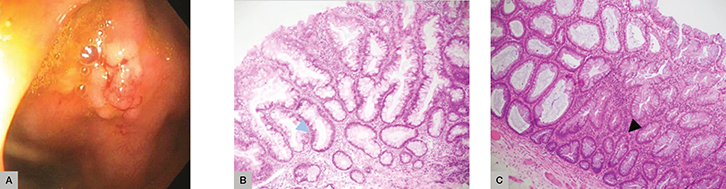 |
Figure 2. Case 2
(A) Endoscopy sessile polyp (B) Glands have serrated lumens extending to the crypts with dilated, ‘boot’-shaped distortion of the basal architecture (blue arrow)
(C) Surface mitoses and focal low-grade dysplasia (black arrow) |
Case 3
Mr CA, aged 59 years, presented to his GP complaining of fatigue. He was found to have iron deficiency anaemia. He had a known family history of CRC and was referred for investigative endoscopy and colonoscopy. An ulcerated mass in the caecum was biopsied. Histopathology revealed an invasive, well-differentiated, low-grade adenocarcinoma arising in a background of dysplastic epithelium (Figure 3). Irregular, dilated glands and small nests of atypical cells were seen infiltrating into the submucosa. Subsequently, Mr CA underwent a right hemicolectomy and is under the care of an oncology team.
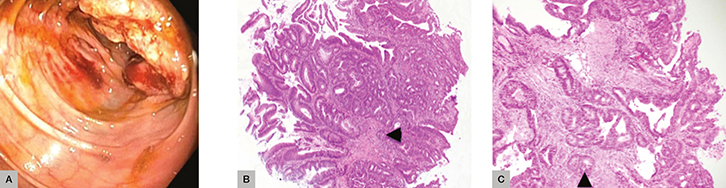 |
Figure 3. Case 3
(A) Endoscopy with caecal mass (B) and (C) Irregular, dilated glands and small nests of atypical cells are seen infiltrating into the submucosa (black arrows) |
Authors
Marjorie M Walker BMedSci, BMBS, FRCPath, FRCPA, AGAF, Professor of Anatomical Pathology, Faculty of Health and Medicine, School of Medicine & Public Health, University of Newcastle, Callaghan, NSW. marjorie.walker@newcastle.edu.au
Angela K Harris BMedSci, MBBS, PhD, Registrar, Pathology North Hunter, John Hunter Hospital, New Lambton Heights, NSW
Georgia C Edwards BSc, MBBS (Hons), Advanced Trainee, Department of Gastroenterology and Hepatology, John Hunter Hospital/Calvary Mater Hospital, New Lambton Heights, NSW
Nicholas J Talley MD, PhD, FRACP, FAFPHM, FAHMS, Pro Vice-Chancellor (Global Research), University of Newcastle, Callaghan, NSW
Competing interests: Outside this work Marjorie M Walker has received consultancy fees from Prometheus, San Diego, USA.
Provenance and peer review: Commissioned, externally peer reviewed.