Combined oral contraceptives (COCs) remain a widely used contraceptive method.1,2 General practitioners commonly see women for contraception-related consultations,1 and therefore must provide evidence-based information about the risks and benefits within the context of the full range of contraceptive options. The rise of social media as a source of medical information makes it important for healthcare professionals to counterbalance emotive misinformation and misperceptions about the risks of COCs, in particular the risk of venous thromboembolism (VTE), with balanced, easy-to-understand facts.
The earliest COCs contained relatively high doses of the synthetic oestrogen, ethinyl oestradiol (EE), in combination with the progestin levonorgestrel or norethisterone. Changes in COC formulations over the past 50 years have included a reduction in EE dose, substitution of EE with oestradiol and use of newer, receptor-selective progestins including desogestrel, gestodene, drospirenone and nomegestrol acetate. These changes have been aimed at reducing cardiovascular risks and troublesome side effects while potentially enhancing desirable ‘non-contraceptive’ effects.
In Australia, the annual incidence of VTE in the general community is approximately 0.6 cases per 1000 population.3 The incidence appears to be increasing in line with other high-income countries, which probably relates to an increase in diagnostic capability as well as increases in rates of modifiable risk factors such as obesity, smoking, long-distance travel and current COC use.4 Major non-modifiable VTE risk factors include increasing age and inherited coagulopathies, in particular factor V Leiden mutation.5 Current low-dose COC use (≤35 µg EE) is associated with an elevated VTE risk of twofold to threefold above baseline, which is highest in the first year of use. For most women of childbearing age, this translates into a very small absolute risk.2,6,7 This absolute risk is also far less than that attributed to pregnancy or the postpartum period (Table 1). An increasing body of international evidence points to the oestrogen component as the major contributor to VTE risk on the basis of the absence of an association for progestin-only contraceptives and an elevated risk for COCs containing more than 35 µg EE.8 However, international controversy remains regarding the influence of different progestins on VTE risk. Conflicting results from several European and US-based studies have led to confusion about whether certain COC formulations can safely be prescribed.9–13
In this paper, we present a systematic review and meta-analysis of all available evidence relating to the risk of VTE associated with drospirenone-containing COCs. We aim to support best-practice prescribing for medically eligible women choosing an oral method of contraception.
Table 1. Risk of VTE in women at various life stages5,6
|
| Population | Risk of VTE
per 10,000 women per year
|
|---|
| Women of childbearing age non-OC users |
4 |
| Women taking COC |
7–10 |
| Pregnant and postpartum women |
20–30* |
|
*Postpartum rates for the first 12 weeks postpartum have been quoted as 40–65 per 10,000 women per year,35 and approximately 300–400 per 10,000 women per year during the two days before and the day after delivery36
COC, combined oral contraceptive; OC, oral contraceptive; VTE, venous thromboembolism
|
Methods
Study
Methods of analysis and inclusion criteria were specified a priori and documented in a protocol. This systematic review was registered on PROSPERO (registration number CRD42014013589).
Data sources
We performed a systematic review of Medline, EMBASE, Derwent Drug File, Biosis, ISI – Current Contents, Chemical Abstracts and the International Pharmaceutical Abstracts, along with the Bayer internal company database. Searches were performed in August 2014 and updated in May 2015. We searched for all clinical studies (prospective or retrospective observational studies or randomised controlled trials) in women taking the COC that compared drospirenone with other progestins, and included information on VTE incidence. We included studies in all languages, but excluded case studies and case series. Searches were independently conducted by two librarians.
Study selection
We identified 43 studies in our search, of which five were duplicates and were excluded. Of the remaining 38 studies, 22 were excluded following abstract review (Figure 1). The included studies underwent quality appraisal using the MERGE criteria.14 One author completed the MERGE assessment, and a second author reviewed the assessment. A third author resolved disagreements between authors. Papers with a high risk of bias (MERGE category ‘C’) were excluded from the narrative review (one paper15 was excluded). The remaining 15 studies were included.9–13,16–25 Additional information for one study was obtained from a Food and Drugs Administration (FDA) briefing document.26 Characteristics of the included studies are reported in Table 2 (available online only).
 |
| Figure 1. PRISMA flowchart |
Data extraction
Data extraction forms were piloted by two authors and modifications were made before sending to all authors for analysis. One author completed data extraction using the pre-defined data fields and a second author reviewed the extraction. A third author resolved disagreements between authors. We extracted information on participant demographics, study setting, comparators and relative risks of VTE. Given an anticipated high level of heterogeneity between the retrieved studies, we did not combine all studies meta-analytically to form one overall pooled risk estimate. We instead combined studies of similar type using Mantel-Haenszel fixed effects meta-analysis to give a pooled estimate for each study type. As some studies reported odds ratios (OR) or relative risks (RR), while others reported hazard ratios (HR), methodological issues may arise with combining these different types of measures, leading to potential bias of the estimate by study group.27 Therefore, a sensitivity analysis was conducted excluding the HR or the OR/RR measure (depending on study type) to explore whether the study type estimates were stable. Heterogeneity was assessed using I2 measures.
Results (data synthesis)
Studies were grouped by type with a pooled estimate for each: prospective cohort (n = 4), retrospective cohort (n = 6), case-control (n = 2), nested case control (n = 3, including non-fatal idiopathic VTE (n = 2), and all VTE (n = 1)).
In the prospective cohort studies, there were no differences in VTE risk between those taking drospirenone-containing COCs, compared with those taking levonorgestrel10,11,18 or other COCs21 (combined RR = 0.94, 95% confidence interval [CI] = 0.75,1.18; Figure 2). This lack of difference remained when excluding the INGENIX study,21 which reported an RR (combined HR = 0.95, 95% CI = 0.74,1.21; Figure 3). No difference in risk was observed in the case control studies9,23 either (combined RR = 1.21, 95%
CI = 0.72, 2.02; Figure 2).
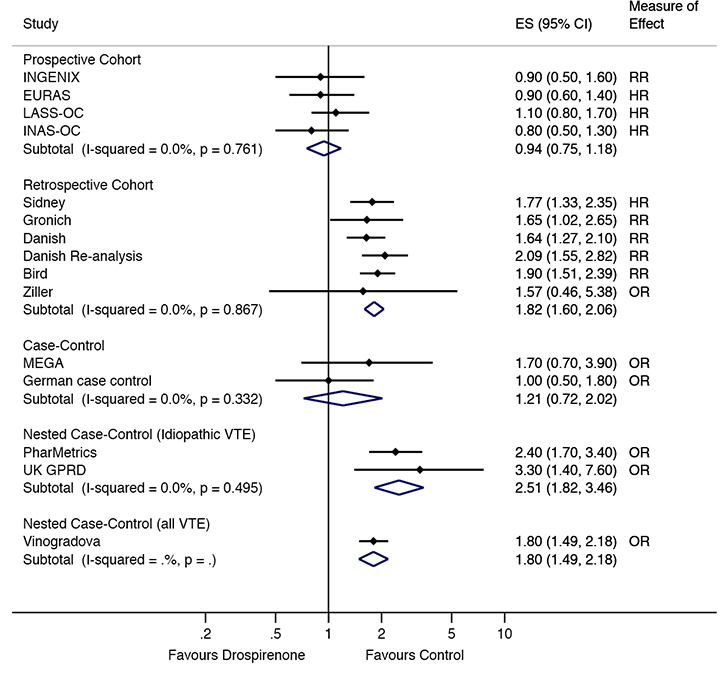 |
| Figure 2. VTE risk in drospirenone users compared to other oral contraceptive users by study type ES, effect size (relative risk, odds ratio or hazard ratio) |
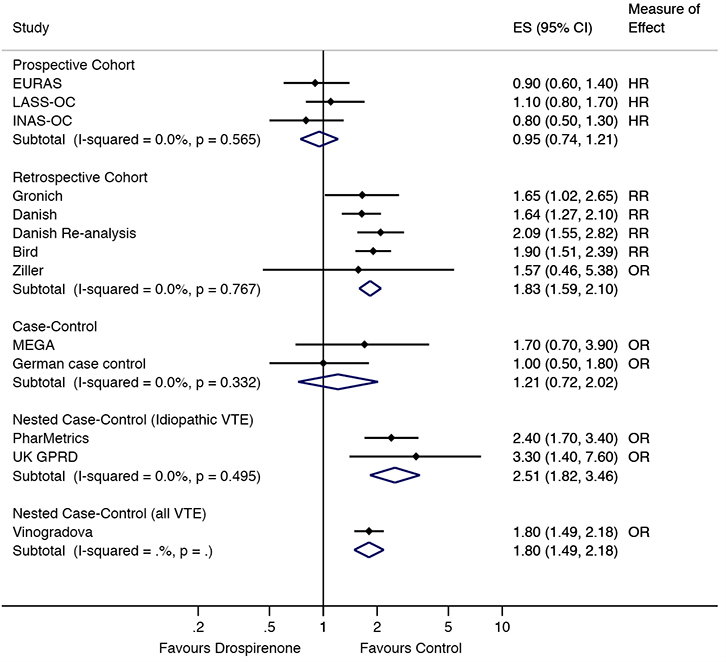 |
| Figure 3. Sensitivity analysis of VTE risk in drospirenone users compared to other oral contraceptive users by study type. ES, effect size (relative risk, odds ratio or hazard ratio) |
The retrospective cohort studies12,13,16,17,22,24 showed a significant increase in risk of VTE in women taking drospirenone-containing COC, compared with women taking levonorgestrel or other COCs (combined RR = 1.82, 95% CI = 1.60, 2.06; Figure 2). This increased risk was still observed when the Sidney study,22 which reported an HR, was excluded (combined RR = 1.83, 95% CI = 1.59, 2.10). An increased risk was also observed in the nested case control studies of idiopathic VTE19,20 (combined RR = 2.51, 95% CI = 1.82, 3.46; Figure 2) and all VTE25 (OR = 1.80, 95% CI = 1.49, 2.18; Figure 2).
Discussion
While current evidence supports a twofold to threefold increase in the risk of VTE for low-dose oestrogen-containing COCs (≤35 µg EE)6 over non-use, the difference in risk between COC types on the basis of the progestin content remains controversial. Our systematic review of all available studies has found consistent differences in VTE outcomes for drospirenone-containing COCs when compared with levonorgestrel and other COCs between studies with a retrospective or nested case control design, but not in case control and prospective cohort studies.
Understanding the difference in VTE risk between COC preparations as well as between studies and study types is challenging for the clinician, and has been the subject of much debate. A randomised controlled trial might settle this issue, but given the rarity of VTE events, it is unlikely that one would ever be completed given the very large sample size that would be required. Restrictive inclusion criteria may also limit the generalisability of the data to everyday clinical practice. There are several known non-modifiable and modifiable VTE risk factors including older age, genetic predisposition, obesity and smoking,28 and it would be important to be able to adjust for these factors when making comparisons between progestins. In line with best-practice prescribing guidelines,2,29 COCs are contraindicated in women with known VTE risk factors, and non–oestrogen-containing alternative methods of contraception should be provided instead.28
Interpretation of the evidence has also been made more complex by phenomena such as an early user effect, reported in some studies, with increased risk of VTE events reported in new users of COCs.11,16,30 The mechanism for this increased risk in new users is poorly understood, but may be due to differential effects on sex hormone binding globulin or activated protein C resistance in early use, or by an unmasking of an underlying inherited coagulation disorder. The effects on coagulation parameters may explain the higher incidence of clotting after a COC break of four weeks or more.31 As user status may be more difficult to determine in retrospective database studies than in studies capturing this information prospectively from patients, it may result in unbalanced comparisons between groups. It should be noted that all of the included studies had limitations in design, including differences in the inclusion criteria. The two large prospective observational studies (INAS-OC and EURAS)10,11 were sponsored by Bayer Healthcare as a requirement of the regulatory bodies. These studies had minimal inclusion/exclusion criteria and any woman who was seeking a new COC or switching COCs was eligible, which meant that some women with higher risk of thrombotic events were prescribed a COC despite not meeting current medical eligibility criteria. Many studies also had significant data limitations in relation to VTE-related risk factors. For example, many of the retrospective studies did not collect information about a current or previous history of VTE, or a family history of VTE.25,28 Studies also did not report the presence of factor V Leiden mutation, which shows marked regional variation. It is estimated to affect 5% of the Australian population.32 Despite the association with VTE, there is no indication for routinely screening women for factor V Leiden mutations prior to COC prescription. The COC is contraindicated in women with a known thrombogenic mutation,33 while caution is needed in those with a first-degree relative who experienced a VTE of any cause before the age of 45 years, as the risks are thought to outweigh the benefits.28,33
The applicability of the study outcomes to the global population is difficult to ascertain. The women in the large prospective observational INAS-OC study were recruited from Europe and the US and, overall, were relatively young (mean age 26.3 years), had a mean body mass index (BMI) of 24.9 kg/m2 and were mainly non-smokers; only 16.9% of the cohort were obese (BMI ≥30 kg/m2) and 22% were smokers. It is difficult to know whether these demographics can be generalised to other COC-taking populations such as Australian women. Unfortunately, information about BMI, obesity and smoking status was not routinely collected in many of the other included studies, particularly in the retrospective cohort studies, or was not captured in the database for all patients in nested case control studies.
Given the differences in results and study methods observed between the prospective and retrospective studies, it is difficult to determine the ‘truth’ in relation to VTE risk between different COC preparations. The retrospective studies had issues such as no validation of reported VTE events, insufficient risk factor data and possible provider bias due to selective prescribing of drospirenone-containing COC in higher risk women. The prospective cohort studies, especially those published more recently, may also suffer from prescriber bias in either direction, possibly due to the media influencing prescribing behaviour. Additionally, one might consider the lack of difference in studies funded by pharmaceutical companies as ‘evidence of influence’, although we note that on investigation these studies appear to be scientifically robust and independent. We cannot, therefore, exclude the possibility that the relative risk of VTE in women taking drospirenone-containing COC lies somewhere between the estimates of the prospective and retrospective analyses.
A strength of our study was the inclusion of the larger prospective studies and the use of adjusted risks where these were available. A recent network meta-analysis by de Bastos and colleagues34 examined VTE risk in women taking COCs. However, their analysis excluded the larger prospective studies (INAS, INGENIX, EURAS or LASS-OC; Table 2, available online only), as they restricted their focus to studies analysing the first VTE event, and the data entered into network meta-analysis was not adjusted for possible confounders. While excluding the prospective studies that included women who had experienced a previous VTE (in whom, therefore, COC use should have been contraindicated) may be seen as a strength of the meta-analysis by de Bastos and colleagues, it also potentially results in a loss of data from the vast majority of women who had not suffered a previous VTE event who were also included in these studies.
When making treatment decisions about prescribing COCs, consistent with evidence-based practice, individual risk factors for each woman must be carefully considered. Prescribers are encouraged to prescribe according to medical eligibility criteria and patient preferences. We believe those women who are eligible for COCs can use any COC with 35 µg of ethinyl oestradiol or less, or the newer oestradiol/oestradiol valerate COCs (which are not included in this review because of lack of data). Those who are ineligible for an oestrogen-containing method due to VTE risk factors, including women with a high BMI and factor V Leiden mutation, should not be prescribed any COC.
Implications for general practice
The risk of VTE for any woman taking a low-dose COC (≤35 µg EE) is approximately two to three times higher than for non-users. In absolute terms, risk of VTE is often quoted in the literature as 4/10,000 women-years for non-pregnant non-users and 7–10/10,000 women years for COC users, which translates to a very low, acceptable absolute risk for any user when appropriate prescribing guidelines are applied. The question of whether or not the risk of VTE among users of drospirenone-containing COCs is higher again cannot be definitively answered by the available scientific literature as the retrospective studies suggest an increased risk, whereas the prospective studies show no difference. However, this does, of itself, suggest that any change in absolute risk in drospirenone-containing COCs must remain extremely small in absolute terms. The choice of contraceptive method should always be made together with the patient, on the basis of the evidence using appropriate medical eligibility criteria. We do not believe the available scientific evidence supports selective prescribing of COC based on ‘differential’ VTE risk alone. COC choice should be based on other factors, including the side effect profile, potential additional benefits and cost. Ultimately, however, the choice of COC type is up to the woman herself with, ideally, expert, up-to-date evidence-based advice from her clinician.
Acknowledgements
The authors would like to thank Sharon Pawelski from SHine SA for conducting the literature searches for this paper.
Author declaration
All authors were involved in critical review of included papers and extraction of data for inclusion in the study. All authors critically reviewed the manuscript and approved it for submission.
Authors
Deborah Bateson MA (Oxon), MSc (LSHTM), MBBS, Medical Director, Family Planning, NSW; Clinical Associate Professor, Discipline of Obstetrics, Gynaecology & Neonatology, University of Sydney, NSW. deborahb@fpnsw.org.au
Belinda E Butcher BSc (Hons) MBiostat, PhD, Director, WriteSource Medical Pty Ltd, NSW
Catherine Donovan BSc(Med)MBBS MMed (Clin Epi) MPH MBA, Associate Medical Director, Bayer Australia Ltd, NSW
Louise Farrell MBBS, RANZCOG, FRCOG, Director of PGME, King Edward Memorial Hospital for Women, WA
Gab Kovacs MBBS (Hons)FRCOG, FRANZCOG, CREI, FAICD, Cert Mgt, Monash University, VIC
Tonia Mezzini BA (Hons), BMBS, MHSSH, FRACGP, FAChSHM, Consultant Sexual Health Physician, Lecturer, Discipline of Rural Health, Adelaide University School of Medicine, SA
Camille Raynes-Greenow BA,MPH, PhD, Grad Dip Pop Hlth, NHMRC Career Development Fellow, Sydney School of Public Health, University of Sydney, NSW
Gino Pecoraro MBBS, FRANZCOG, Lecturer, Department of Obstetrics and Gynaecology, University of Queensland, QLD
Christine Read MBBS, Grad Cert Public Health, Women’s Health Matters, Lismore, NSW
Rod Baber MBBS, BPharm, FRCOG, FRANZCOG, Clinical Associate Professor, Discipline of Obstetrics, Gynaecology & Neonatology, University of Sydney, NSW
Competing interests: In relation to this work, the organisations of Deborah Bateson, Belinda E Butcher, Gab Kovacs, Tonia Mezzini and Rodney Baber have been paid consulting fees and have received travel and/or administrative support from Bayer Healthcare. Louise Farrell, Camille Raynes-Greenow, Gino Pecoraro and Christine Read received personal fees from Bayer in relation to the project.
Outside this work, Deborah Bateson’s organisation has received consultancy fees from Bayer Healthcare and MSD. Belinda E Butcher’s organisation has received consultancy fees and payment for manuscript preparation from multiple pharmaceutical and academic research organisations including Bayer Australia Pty Ltd; her organisation has also been paid by Drive Time Radio and Janssen-Cilag Australia for educational presentations. Catherine Donovan is a medical employee of Bayer Australia. Gab Kovacs has had expenses covered by Bayer, MSD, Merck and Serono. Tonia Mezzini’s organisation has been paid by Bayer for educational presentations. Gino Pecoraro has had expenses covered by Bayer, MSD, Lilly, Organon and Ferring. Christine Read has been paid consultancy fees and has had expenses covered by Bayer, Novo Nordisk, Pfizer and MSD. Rodney Baber’s institution has received grants from Organon, Pfizer and Novartis, and lecture and educational presentations payments from Merck, Abbott and Bayer.
Provenance and peer review: Not commissioned, externally peer reviewed.
Table 2. Characteristics of included studies
|
| Source | Study
type | Setting | Number of eligible patients | Age, years; mean (SD) or (min to max) |
|---|
| INGENIX20 |
P-CS |
US claims database |
DRSP: 22,429
Comparator: 44,858 |
28.4 (NR)
28.4 (NR) |
EURAS11
|
P-CS |
Europe &
UK community |
DRSP: 16,534
LNG: 15,428
OCOC: 26,341
NOHC: 371 |
25.9 (8.1)
25.1 (8.7)
24.8 (7.8)
27.5 (8.0) |
| LASS-OC18,26‡ |
P-CS |
Europe community |
47,799 |
NR |
| INAS-OC10 |
P-CS |
US and Europe community |
85,109 |
26.3 (7.7) |
| Sidney22 |
R-CS |
|
DRSP: 109,070
Comparator: 383,151 |
26.4§
27.2 |
| BMI | Obese (%)* | Ever used
OC pill
(%) | Current users of OC pill (%) | Family history
of VTE (%) |
|---|
NR
NR |
NR
NR |
100
100 |
100
100 |
NR
NR |
22.9 (4.4)
22.0 (4.0)
21.7 (3.8)
21.7 (3.7) |
8.2
5.2
4.5
NR |
NR
NR
NR
NR |
100
100
100
NR |
0.8†
0.6†
0.8†
1.6† |
| NR |
NR |
NR |
NR |
NR |
| 24.9 (5.9) |
16.4 |
100 |
100 |
2.45 |
NR
NR |
NR
NR |
100
100 |
100
100 |
NR
NR |
Personal history
of VTE (%) | Comparisons
considered | Follow-up or study period | MERGE |
|---|
0.1
0.1 |
DRSP versus
other COC |
14,081
women-years |
B1 |
NR
NR
NR
NR |
DRSP versus
LNG/OCOC |
142,475
women-
years |
B1 |
| NR |
LNG versus DRSP versus OCOC |
2000–10; 318,784 women-years |
B1 |
0.12 (DVT)
0.03 (PE) |
LNG versus DRSP 24d or 21d versus OCOC |
2002–11; 206,296 women-years |
B1 |
NR
NR |
DRSP versus
other COC|| |
2001–07 |
B1 |
*Where not explicitly stated, a BMI over 30 kg/m2 was considered obese
†VTE-like conditions
‡The LASS-OC study is an extension of EURAS, so the demographics between the two studies would be very similar
§Mean age at initiation for all users (including comparator, norelgestromin-containing transdermal patch and tonogestrel vaginal ring)
||LNG10-20; LNG15-30; norethindrone 1mg/ethinyl estradiol 20 μg; and nogestimate (0.18–0.25 mg)/ethinyl estradiol 35 μg |
Table 2. Characteristics of included studies
|
| Source | Study
type | Setting | Number of eligible patients | Age, years; mean (SD) or (min to max) |
|---|
| Gronich14 |
R-CS |
Israel Clalit medication database |
329,995 |
NR (12 to 50) |
| Danish12 |
R-CS |
Danish census (community) |
NR |
NR, but most WY of exposure in 35–39 year olds |
| Danish re-analysis13 |
R-CS |
Danish census (community) |
1,296,120 |
15 to 49 |
| Bird16 |
R-CS |
US community |
DRSP/EE20: 75,524
LNG/EE20: 111,151
DRSP/EE30: 163,159
LNG/EE30: 82,344 |
30.0 (NR)
28.1 (NR)
28.2 (NR)
30.0 (NR) |
| BMI | Obese (%)* | Ever used
OC pill
(%) | Current users of OC pill (%) | Family history
of VTE (%) |
|---|
| NR |
NR |
NR |
100 |
NR |
| NR |
NR |
52.9# |
31.1# |
NR |
| NR |
NR |
68.3 |
NR |
NR |
NR
NR
NR
NR |
11.22
10.41
11.40
11.68 |
100
100
100
100 |
100
100
100
100 |
NR
NR
NR
NR |
Personal history
of VTE (%) |
Comparisons considered |
Follow-up or study period |
MERGE |
| NR |
2nd generation versus 3rd generation versus DRSP versus gestodene versus chlormadinone |
2002–08; 819,749 women-years |
|
| ?** |
LNG versus DRSP versus OCOC |
1995–2005; 10.4 million women-years |
B2 |
| NR |
DRSP versus LNG and 3rd generation COC |
2001–09; 8.0 million women-years |
B1 |
NR
NR
NR
NR |
DRSP/EE20 versus LNG/EE20
DRSP/EE30 versus LNG/EE30 |
2001–09 |
B1 |
#Proportion of women-years of follow-up
**Women with malignancy and CVD ICD codes were excluded, although it is unclear whether these exclusions would have captured all women with a personal history of VTE |
Table 2. Characteristics of included studies
|
| Source | Study
type | Setting | Number of eligible patients | Age, years; mean (SD) or (min to max) |
|---|
| Ziller24 |
R-CS |
SP |
68,168 |
29.6 |
| MEGA23 |
C-CS |
Anticoagulati
on clinics
Netherlands |
Case: 1,524
Control: 1,760 |
37.1 (18 to 49)
37.4 (18 to 49) |
German case
control9
Vinogradova25 |
C-CS
N-CC
All VTE |
German community
CPRD database
QResearch database |
Case: 680 Control: 2,720
Cases UK: 5,062
Control UK: 19,638
Cases QR: 5,500
Control QR: 22,396 |
36.1 (9.0)
36.1 (9.0)
15 to 49 |
| PharMetrics19 |
N-CC
I |
US claims
database |
Case: 681
Control: 186 |
15 to 44
15 to 44 |
| UK GPRD20 |
N-CC
I |
UK GP database |
Case: 61
Control: 215 |
32.2 (7.2)
31.8 (7.4) |
| BMI | Obese (%)* | Ever used
OC pill
(%) | Current users of OC pill (%) | Family history
of VTE (%) |
|---|
| 23.5 |
NR |
100 |
100 |
NR |
26.8 (16
to 57.8)
24.4 (15.7
to 50.7) |
NR
NR |
NR
NR |
72.4
37.4 |
26.4
14.3 |
NR
NR
15 to ≥30 |
21.2
13.3
12.5
16.3
19.3
21.6 |
84.7
80.8
NR
|
40.1
29.9
32.6
20.3
33.4
19.7
|
30.6
12.9
NR
|
NR
NR |
13
6 |
100
100 |
100
100 |
NR
NR |
26.1 (19.1 to 45.7)
23.3 (17.3 to 43.2) |
25
7 |
100
100 |
100
100 |
excl.
excl |
Personal history
of VTE
(%) | Comparisons considered | Follow-up or study period | MERGE |
|---|
| 0 |
LNG versus DRSP |
2005–10 |
B2 |
NR
NR |
COC versus NCOC Progestogen type; estrogen type |
1999– 2004 |
B2 |
7.4
2.1
NR
|
LNG versus
DRSP
LNG versus DRSP
|
2002–08;
2001–13
|
B1
B1 |
NR††
NR |
LNG versus
DRSP |
2002–08 |
B1 |
0
0 |
LNG versus DRSP |
2002–09 |
B1 |
| C-CS, case-control study; COC, combined oral contraceptive; DRSP, drospirenone containing COC; excl, excluded; GP, general practitioner; I, idiopathic VTE study; NCC, nested case-control; NCOC, no combined oral contraceptive; NOHC, non-oral hormonal contraceptive; NR, not reported; OCOC, other COC; P-CS, prospective cohort study; R-CS, retrospective cohort study; SP, specialist practice††Women were excluded if they had important risk factors, including major surgery, lower limb injury, trauma and pregnancy |