Urinary tract infections (UTIs) are common in childhood. An estimated 2% of boys and 8% of girls will experience a UTI by seven years of age, and 7% of febrile infants will have a UTI.1,2 Paediatric UTIs, especially in young children, have varied and non-specific presentations that can be undetected or misdiagnosed. Delaying diagnosis and management of UTIs may potentially result in renal damage and loss of renal function. The aim of this article is to provide clinicians with an overview of the assessment and management of children with UTIs.
Pathogenesis of UTI
Often, UTIs develop when uropathogens ascend from periurethral colonisations to the bladder (cystitis). From the bladder, uropathogens may ascend the urinary tract (pyelonephritis) or invade the bloodstream (urosepsis). UTIs resulting from haematogenous and direct invasion seldom occur. Urine is sterile, but uropathogens may gain entry during catheterisation, turbulent voiding patterns, sexual intercourse or genital manipulation. UTI susceptibility is determined by bacterial virulence, anatomical variances (gender, vesicoureteral reflux [VUR], circumcision), bowel or bladder dysfunction resulting in urinary stasis (constipation and neurogenic bladder), and host defences (genetics and flora of periurethral and gastrointestinal tracts).
In the first year of life, UTIs are more common in boys than girls and 10 times higher in uncircumcised boys, compared with circumcised boys.3 The incidence of UTIs falls below 1% in school-aged boys and increases to 1–3% in school-aged girls.4 Sexual activity increases the risk of UTI in teenage girls.5 Children who have had a UTI have a 13–19% increased risk of recurrence and 17% will develop renal scarring.6 However, few children (<4%) will develop end-stage renal failure from a UTI; this is often a result of recurrent UTIs or VUR.7
Pathogens
Most UTIs are caused by Gram-negative bacteria, of which Escherichia coli is the most common (>75% of UTIs).8,9 Other bacteria causing UTIs include Proteus, Klebsiella, Enterobacter, Citrobacter, Enterococcus and Staphylococcus saprophyticus. Fungal UTIs, such as Candida albicans infections, often coincide with recent antibiotic treatment, urinary catheterisation or immunosuppression.10 Symptomatic viral UTIs are rare; however, adenoviruses are known to cause haemorrhagic cystitis and BK virus (polyoma virus) is a causative organism associated with immunosuppression.11–13
Classification of UTIs
UTIs are classified clinically (asymptomatic versus symptomatic), anatomically (cystitis versus pyelonephritis) and by incidence (single versus recurrent). Recurrent UTIs are often the result of:14
- inadequate antimicrobial therapy
- non-compliance
- bacterial resistance
- host susceptibility
- factors contributing to urinary stasis.
Clinical signs and symptoms
The clinical presentation varies and is often non-specific, particularly in young infants. This makes early diagnosis and management of paediatric UTIs challenging. Therefore, UTIs should be suspected in every febrile infant until proven otherwise.
History-taking includes an antenatal history, along with a family history of urological abnormalities, especially VUR. A full voiding history should include frequency, urgency, stream, volume, suprapubic pain, dysuria, secondary enuresis and toileting practices. Other relevant history includes the amount of fluid intake and bowel habits. In younger children, carers may report non-specific symptoms such as lethargy, fever, vomiting, malaise, failure to thrive, irritability and offensive urine.
No physical sign is pathognomonic for a UTI. On examination, physicians should promptly assess if the patient appears ‘sick’ or ‘well’, and be suspicious of fever, hypertension, a palpable bladder, dribbling or straining, and loin or suprapubic tenderness. Although often unremarkable, physical examination should include assessment of the abdomen, external genitalia, lower limbs and hydration status. In rare instances, underlying conditions that contribute to UTIs, such as spina bifida, phimosis, labial adhesions or sexual abuse, may be present.
Investigations
Urine collection, urinalysis and cultures
Urine should be collected if there is an unexplained fever (>38°C) and/or symptoms suggestive of a UTI.7,15 Collection of uncontaminated urine samples can be challenging in infants and should be performed using one of these methods (Table 1):
- clean catch (CCU)
- mid-stream (MSU)
- catheterised specimen (CSU)
- suprapubic aspiration (SPA).
Bagged urine specimens are often contaminated. A negative sample may exclude a UTI, but all positive bagged urine results should be confirmed using a CCU, MSU, CSU or SPA sample before commencing treatment.16 Urine samples should be collected prior to antibiotic administration to prevent false negative results.
Urinalysis is a quick, non-invasive method to screen for UTIs. However, urinalysis alone is not sufficient to diagnose a UTI. Positive readings for nitrite (75% UTI probability) and leukocyte esterase (30% UTI probability) may suggest a UTI. Urinalysis has 82.5% sensitivity, 81.3% specificity, 33.9% positive predictive value and 97.6% negative predictive value.17 In febrile children, urinalysis can help to identify who should receive antibacterial treatment while cultures are pending.
Diagnosis
UTI diagnosis is based on clinical symptoms in association with a positive urine culture.15,18 The amount of bacterial growth required for a positive culture varies by age and method of urine collection (Table 1). Even though treatment may begin prior to receiving culture results, the causative organism and antibiotic sensitivity should be evaluated to formulate a targeted therapeutic regimen.
Urinary tract imaging
In most circumstances, urinary tract imaging is not recommended following the first UTI.15 Renal ultrasonography seldom provides information that alters management. Clinicians should be aware of the indications and limitations of urinary tract imaging and use clinical judgement when seeking further imaging. Table 2 summarises the indications, uses and limitations of common urinary tract imaging modalities.
Management of UTIs
Treatment and care involves good communication between healthcare professionals, children and carers. Gillick competent children should be involved in the management of their health.19 Figure 1 provides an algorithm for the routine medical management of paediatric UTIs.
Conservative management
A positive urine culture in the absence of clinical symptoms may indicate asymptomatic bacteriuria and does not warrant treatment or further investigation. For all children, general measures that improve hygiene, hydration and bowel habits are recommended.7,15
Medical management
Treatment should be tailored to clinical severity and depends on the child’s age. Broad spectrum oral antibiotics will treat most uncomplicated UTIs. Comparatively, children with apparent sepsis, in shock and/or <3 months of age should be treated aggressively with parenteral antibiotics and intravenous fluids. These patients should be referred to hospital for a full septic screen, including lumbar puncture and paediatric review.7,15 Antibiotic choice is governed by microbial sensitivities and local policies (Table 3, available online only). Every patient should be reassessed 48 hours after starting antibiotics, and treatment should be modified as per cultures and sensitivities. Empirical gentamicin therapy should not be used for longer than three days. If empirical therapy is still required, switching to ceftriaxone should be considered to reduce the risk of nephrotoxic and ototoxic side effects.20
Surgical management
Evidence suggests that boys have a 1% UTI risk in their first year, but this risk is reduced to 0.1% if they are circumcised.2 Routine circumcision is not recommended, given that approximately 111 boys would need to be circumcised to prevent one UTI. However, having already had a first UTI in the first year of life confers further risk and circumcision may provide additional benefit, especially for those with recurrent UTI or grades III–V VUR.15 Prior to circumcision, hypospadias should be evaluated. Furthermore, surgical VUR correction should be considered only for persistent grade III–V VUR and/or failed continuous antibiotic coverage.15,21
Alternative management
The evidence indicates that cranberry concentrates effectively treat UTI symptoms in adults. However, there is no evidence to suggest that cranberry concentrate is therapeutic, prophylactic or reduces UTI symptoms in children, and is not recommended.22
Prevention and follow-up
According to Australian guidelines, antibiotic prophylaxis is not recommended for children after a first UTI.15 Instead, antibiotic prophylaxis should be considered for VUR grades III–V and/or complicated, recurrent UTIs. This decision should be made by a specialist or general practitioners specialising in paediatric care.1,23 When trialling antibiotic prophylaxis, continuation should be reviewed every six months. In addition, conservative measures such as increasing fluid intake, avoiding bubble baths, improving hygiene, and addressing constipation and dysfunctional voiding issues should be addressed to limit recurrence.24
Infants generally do not require follow-up if they have had asymptomatic bacteriuria or normal imaging. Children with recurrent UTIs should be assessed by a paediatrician and may require additional imaging, blood pressure monitoring and proteinuria assessment. Infants with impaired renal function or bilateral renal abnormalities require close paediatric involvement, annual blood pressure monitoring, renal imaging
and renal function tests. It is import to note that any febrile event in these children
needs to be investigated with urine cultures.7
Table 1. Urine specimen collection methods7,15
| | Urine bag | Clean catch (CCU/MSU) | Catheterisation (CSU) | Suprapubic aspiration (SPA) |
|---|
|
Description
|
After careful cleaning of the perineum, an adhesive plastic bag is applied to collect urine
|
The stream of urine is caught in a urinary specimen pot. The risk of contamination is reduced by:
- cleansing the perineum and meatus prior to collection with anti-septic solution
- retracting the labia and foreskin
- collecting a midstream sample
|
A midstream urine sample is collected using in and out catheterisation. Refer to paediatric tables for appropriate catheter sizes. Estimated size is 2x ETT size. Lignocaine 2% gel aids in insertion but takes 10 minutes for full analgesic effect. Consider nitrous oxide or sucrose analgesia in young infants
|
Under ultrasound guidance, a 1.5 inch, 22 G needle is attached to 5 ml syringe and inserted into a full bladder (>20 mL or >1 cm in all axes US scan). The bladder lies midline approximately 1–2 cm above the pubic symphysis. The procedure is relatively safe and complications are rare. The patient must be held on their back and restrained by their parent to provide adequate access to the bladder. Light sedation may also be needed
|
|
Indications
|
Unable to collect urine by alternative methods
|
- Children capable of voiding on request
- Parental concerns regarding CSU and SPA collections
|
- Infants unable to provide CCU specimen
- Concerns of CCU contamination
- Acute urinary retention
|
- Infants unable to void on request
- Uncircumcised boys with redundant foreskin or phimosis
- Girls with labial adhesions
- Periurethral irritation
|
|
Contamination
|
Highest rate of contamination: false positive rate 88–99%
|
Potential risk of white cell and bacteria contamination from foreskin and reflux of urine into the vagina. Greater risk of contamination than CSU
|
Potential risk of white cell and bacteria contamination from foreskin and reflux of urine into the vagina
|
Skin flora contamination is rare
|
|
UTI diagnosis
|
- High contamination rate
- Not suitable to diagnose a UTI
|
>105 CFU/mL clinically relevant organisms + pyuria/bacteruria*
|
Any growth of clinically relevant organisms + pyuria/bacteruria*
|
|
Benefits
|
- Non-invasive technique
- Negative culture tests may exclude a UTI
|
- Non-invasive technique
- Preferred technique for infants able to void on request
|
Presumed to be less painful and less invasive than SPA
|
- Preferred aseptic method
- Less likely to acquire contamination
|
|
*Lactobacillus spp; Coagulase (–) Staphylococci and Corynebacterium spp are not considered clinically relevant bacteria
CCU, clean catch urine; CSU, catheterisation; MSU, mid-stream urine; SPA, suprapubic aspiration; UTI, urinary tract infection
|
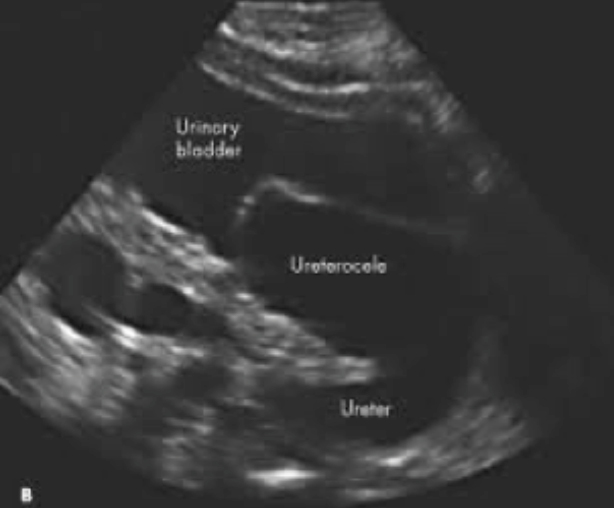
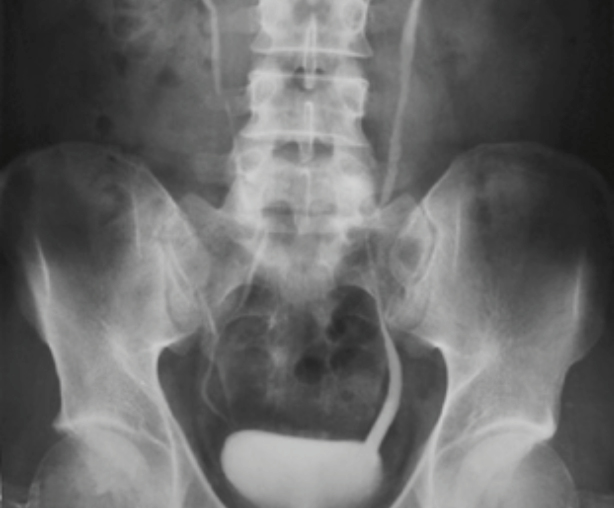
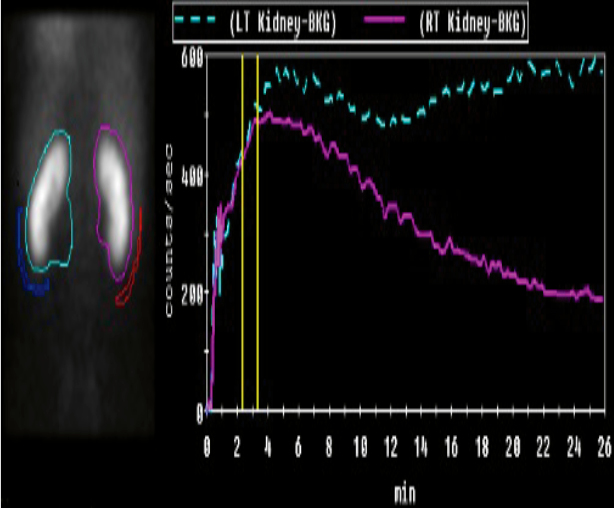
Table 2: Renal imaging modalities
|
|
|
|
|
KUB ultrasound
First-line, non-invasive imaging
|
MCUG
Contrast radiographic imaging
|
Nuclear scans DMSA and MAG3Radioisotope nuclear imaging
|
|
Uses
|
|---|
|
Assess
- Fluid collections
- Bladder volume
- Kidney: size, shape, location
- Urinary tract: obstructions, dilatations
|
Confirm
- Posterior urethral valves
- Obstructive Uropathies
- Gold standard for VUR diagnosis
|
Confirm
Suspicion of renal damage
DMSA: Gold standard for renal scar detection
MAG3:
- Faster, less radiation
- Renal excretion enables micturition study
|
|
Indications
|
|---|
- Concurrent bacteraemia
- Atypical UTI organisms
- Staphylococcus aureus
- Pseudomonas
- UTI <3 years old
- Non/inadequate response to 48hrs of IV antibiotics
- Abdominal mass
- Abnormal voiding
- Recurrent UTI
- First febrile UTI and no prompt follow up assured
- Renal impairment
- Significant electrolyte derangement
- No antenatal renal tract imaging in second to third trimester
|
- Abnormal renal ultrasound
- Hydronephrosis
- Thick bladder wall
- Renal scarring
- Abnormal voiding post-febrile UTI
- Post-second febrile UTI
- Suspicion of
- VUR
- posterior urethral valves
|
- Clinical suspicion of renal injury
- Reduced renal function
- Suspicion of VUR
- Suspicion of obstructive uropathy on ultrasound in older toilet-trained children
|
|
Limitations
|
|---|
- Does not asses function
- Operator dependent
- Cannot diagnose VUR
|
- Radiation exposure ~1 mSv
- Invasive
- Unpleasant to perform post-infancy
- May require sedation
- Requires prophylactic antibiotics
|
- Dynamic renal excretion study requires toilet training
- False positives if <3 months post-UTI, therefore can’t use in acute phase (0–4 weeks)
- May require sedation
- Cannot determine old versus new scarring
|
|
DMSA, dimercaptosuccinic acid -99mTc; KUB, kidneys, ureter and bladder; MAG3, mercapto-acetyl-triglycine-99mTc; MCUG, micturition urethrogram; mSv, millisievert; US, ultrasound; UTI, urinary tract infection; VUR, vesicoureteral reflux
|
Table 3: Oral antibiotic regimens for paediatric UTIs as listed in the electronic Therapeutic Guidelines19
|
eTG listed
|
Antibiotics
|
Therapeutic dose
|
Prophylactic dose
|
Adverse effects
|
Targets
|
Notes
|
|---|
|
1.
|
Trimethoprim (TMP)
‘Alprim’
|
- 4 mg/kg BD
Max: 150 mg BD
|
- 4 mg/kg Nocte
Max: 150 mg Nocte
|
- Nausea and vomiting
Pruritus
Rash, Stevens-Johnson syndrome
Hyperkalaemia
Thrombocytopenia
Leucopenia
|
- Escherichia coli
Enterobacter spp.
Klebsiella spp.
Proteus mirabilis
Coagulase (–) Staphylococcus aureus
|
- Contraindicated in renal impairment and folate deficiency
Multiple drug interactions
Not recommended for children younger than 1 month
|
|
|
Trimethoprim–sulfamethoxazole (TMP–SMX)
‘Bactrim’
|
- 4 + 20 mg/kg BD
Max: 16 0+ 180mg BD
|
- 2 + 10mg/kg Nocte
Max: 80 + 400mg Nocte
|
- Same as TMP
Hepatotoxicity
Seizures, vertigo
Peripheral neuropathy
Kernicterus
|
- Same as TMP
Broader coverage of Proteus & Morganellea spp.
|
- Can lead to Pseudomembranous colitis
Causes haemolysis in G6PD deficiency
Not recommended for children younger than 1 month
|
|
2.
|
Cephalexin
‘Keflex’
|
- 12.5mg/kg QID
Max: 500 mg QID
|
- 12.5 mg/kg Nocte
Max: 25 0mg Nocte
|
- Nausea and vomiting
Cholestatic hepatitis
Neurotoxicity
Blood dyscrasia
|
- E. coli
P. mirabilis
Klebsiella spp.
|
- Prevalence of resistance varies by geography
Risk of C. difficile, Candida and Enterococcus spp. infections
|
|
3.
|
Amoxycillin and Clavulanic acid
‘Augmentin’
|
- 22.5 + 3.2 mg/kg BD
Max: 875 + 125 mg BD
|
- Not Recommended for prophylactic UTI treatment
|
- Rash
Transient deranged liver enzymes
Nausea, vomiting, diarrhoea
Cholestatic hepatitis
Electrolyte disturbances
Neurotoxicity
Blood dyscrasia
|
Useful against b-lactamase strains of E. coli, Enterobacter spp. and Klebsiella spp.
|
- Take with meals to enhance absorption
Rashes are often associated concurrent infectious mononucleosis and/or leukaemia
Risk of C. difficile, Candida and Enterococcus spp. infections
|
|
4.
|
Norfloxacin
‘Noroxin’
|
- 10 mg/kg BD
Max: 400 mg BD
|
- Not Recommended for prophylactic UTI Treatment
|
- Rash, pruritus
Nausea, vomiting, diarrhoea
Phototoxicity
Hearing loss and diplopia
Peripheral neuropathy
Tendon rupture
|
- Pseudomonas spp.
Antibiotic resistant bacteria
|
- Causes haemolysis in G6PD deficiency
|
|
5.
|
Nitrofurantonin
‘Macrodantin’
|
- Not recommended for therapeutic UTI treatment
|
- 1 mg/kg Nocte
Max: 50 mg Nocte
|
- Nausea, vomiting, diarrhoea
Rash
Vertigo
Peripheral polyneuropathy
Urine discolouration
Hepatotoxicity is rare
Pulmonary toxicity is rare
|
Gram negative and Gram positive coverage
|
- Not recommended for children younger than 1 month
Causes haemolysis in G6PD deficiency
Antacids reduce potency of drug
|
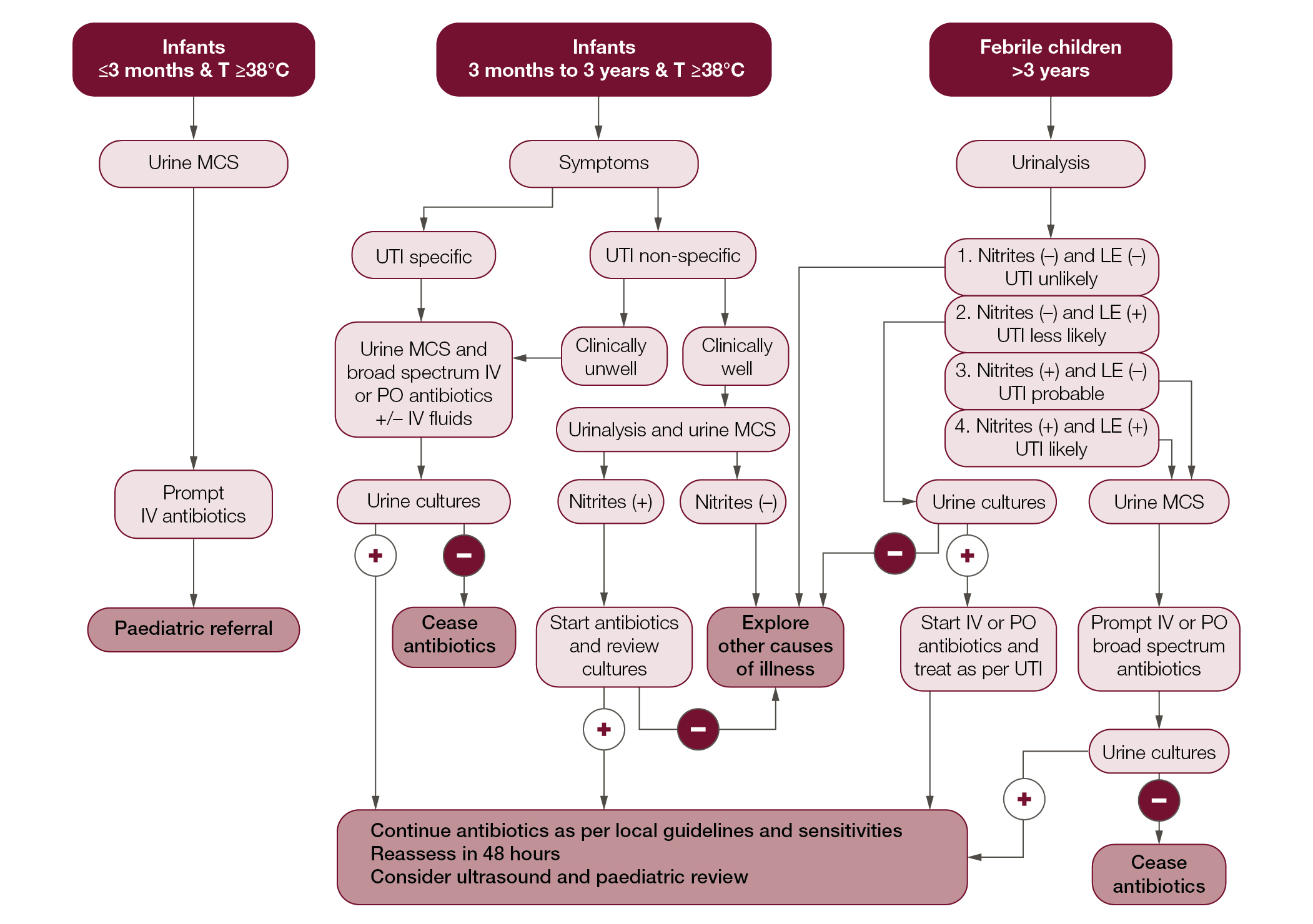 |
| Figure 1. Algorithm for UTI management in children – Devised from the National Institute for Health and Care Excellence and Kidney Health Australia Guidelines6,14 |
Conclusion
UTIs are a common cause of childhood illness. They pose a risk for renal scarring and may potentially contribute to lifelong morbidity from hypertension and chronic renal failure. Appropriate UTI diagnosis and management are important. Management is aimed at treating the acute episode, identifying the aetiology and preventing recurrences. Sterile urine specimen collection is fundamental for diagnosis. When indicated, renal ultrasonography can prevent recurrent UTIs by identifying structural abnormalities that require subsequent renal imaging and further intervention. In most instances, antibiotic prophylaxis and surgical intervention are not required to prevent UTIs. Rather, good hygiene, prevention of constipation, adequate fluid intake and complete bladder emptying can help to prevent most recurrences.
Authors
Devang J Desai MBBS, Urology Registrar, Greenslopes Hospital and Princess Alexandra Hospital, and School of Medicine, University of Queensland, Brisbane, Qld
Brent Gilbert MBBS, MSc, Urology Resident, Greenslopes Hospital and School of Medicine, University of Queensland, Brisbane, Qld. brentgilbert@gmail.com
Craig A McBride MBBS, FRACS, Consultant Paediatric Urological Surgeon, University of Queensland, Lady Cilento Hospital and Royal Womens Hospital, Brisbane, Qld
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer reviewed.