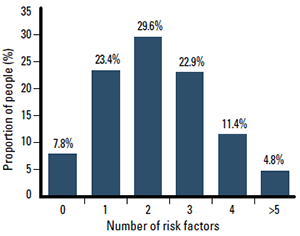
Figure 1. Multiple CV risk factors are common in Australian adults, with 2 in 3 adults having two or more risk factors2
The goal of treating a patient with hypertension is to reduce the risk of fatal and non-fatal CV events and should be based on the available evidence. The most reliable data come from high quality randomised trials and meta-analyses. Despite this, agents with a limited evidence base are often recommended interchangeably with those where clear evidence of benefit has been established. Pharmaceutical Benefits Scheme data show that prescriptions for ARBs are increasing more than ACEIs and other classes for the treatment of hypertension.4 The evidence for ACEIs and ARBs is quite different, and therefore applying this evidence to all patients, including those with hypertension, is problematic.
Mechanism of action
ACEIs and ARBs both act on the renin-angiotensin system (RAS), but in very different ways.5,6 ACEIs decrease synthesis of angiotensin 2 by inhibiting the actions of angiotensin converting enzyme (ACE), whereas ARBs bind directly to one type of angiotensin receptor, AT-1, preventing its activation by angiotensin 2 (Figure 2). The RAS is complex, therefore the effects of ACE inhibition and angiotensin receptor blockade may differ. A number of components to the RAS are not angiotensin receptor dependent. In particular, bradykinin leads to an increase in nitric oxide levels,5 and the recently described peptide angiotensin-(1-7) exerts its effects via the Mas receptor.7,8 Manipulation of non-angiotensin receptor dependent parts of the RAS system may explain why ACEIs potentially reduce CV morbidity and mortality beyond blood pressure regulation.5,6
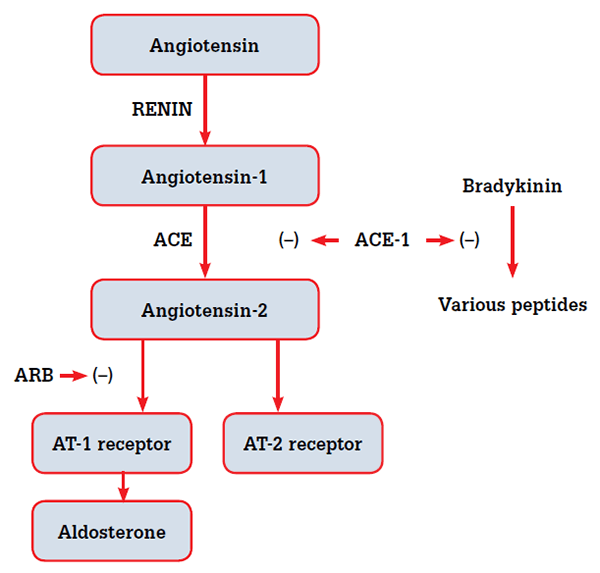
Figure 2. ACEIs and ARBs act on different parts of the renin-angiotensin system and may be expected to have different biochemical and clinical effects
Evidence for ACEI use
The landmark HOPE9 and EUROPA10 trials demonstrated that ACEI therapy prevents CV events and death in patients with multiple CV risk factors or coronary artery disease. ACEIs have consistent clinical trial evidence of mortality benefit in patients at risk of CV events. A meta-analysis of 39 randomised controlled trials involving 150 943 patients reported that ACEI therapy is significantly superior to placebo in reducing the relative risk of death from any cause, CV death, stroke and myocardial infarction (MI).6 When compared against placebo or active alternative drug treatment (including ARBs), ACEIs significantly reduce the relative risk of death from any cause by 9% (p<0.001), CV death by 12% (p<0.001), and MI by 14% (p<0.001) (Figure 3).6 The relative risk of stroke was similar compared with other active treatment (p=0.31), but was reduced by 17% compared with placebo (p<0.05).6 This meta-analysis demonstrates that ACEIs and ACEI combinations are an effective evidence based way to initiate CV risk reduction in patients with hypertension.
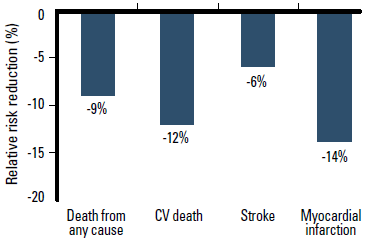
Figure 3. ACEI therapy reduces the relative risk of death from any cause, CV death and myocardial infarction compared with placebo or active treatment6
In patients with chronic heart failure (CHF), ACEIs have been shown to prolong survival in patients with NYHA Class II, III and IV symptoms, compared to placebo.11–13 They also improve symptom status, physical activity, exercise tolerance and the need for hospitalisation in patients with worsening CHF,13,14 and increase ejection fraction compared to placebo.11,13 Studies comparing the use of ACEIs and angiotensin 2 receptor antagonists in heart failure show similar outcomes.13,15 Angiotensin 2 receptor antagonists have been shown to provide additional morbidity and mortality benefits in patients receiving ACEIs for CHF,16 but not for heart failure after acute MI.17 The effect of angiotensin 2 receptor antagonists on mortality alone was not significant in individual trials.13 In patients who are ACEI intolerant, angiotensin 2 receptor antagonists provide morbidity benefits and a non-significant trend toward mortality benefits in comparison to placebo. Therefore, angiotensin 2 receptor antagonists are recommended as an alternative for patients who experience ACEI-mediated adverse effects, such as cough.13
Evidence for ARB use
Clinical trial data for ARBs are less consistent, particularly regarding mortality benefit. A meta-analysis published in 2011, of 37 randomised controlled trials involving 147 020 patients, reported that ARBs reduce the relative risk of stroke by 10% (p=0.007), heart failure by 13% (p<0.001), and new onset diabetes by 15% (p=0.003) compared with placebo or active treatment. However, ARBs do not reduce the risk of MI (1% reduction, p=0.055), CV death (1% reduction, p=0.090) or death from any cause (no reduction, p=0.482) (Figure 4).18 This meta-analysis included some of the more recent studies, particularly the ONTARGET trial that demonstrated that the ARB telmisartan was not inferior to the ACEI ramipril for reducing the risk of major CV events overall, including CV death.19
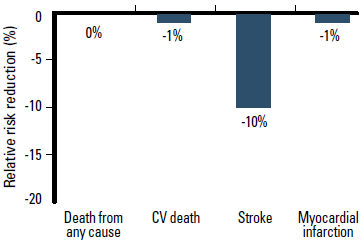
Figure 4. ARB therapy reduces the relative risk of stroke, but not death from any cause or CV death compared with placebo or active treatment18
A pooled analysis, published in 2012, of 20 CV morbidity and mortality trials, where 158 998 patients were randomised to an inhibitor of the RAS system or control, showed a 5% reduction in all-cause mortality and a 7% reduction in CV mortality driven entirely by ACEIs, which were associated with a significant 10% reduction in all-cause mortality, while there was no mortality reduction demonstrated with ARBs.20 Importantly, the treatment effect between ACEIs and ARBs on all-cause mortality was statistically significantly different,20 however, these trials were not direct comparisons. Indirect comparisons between studies are problematic as other factors such as co-prescribed therapies, may confound the comparison of the two sets of trials. Comparisons between trials are also subject to epidemiological fallacies and, where available, head-to-head comparisons are preferred.
This meta-analysis20 can be considered particularly relevant, however, as it included trials in which the majority of patients were hypertensive – the population in which ACEIs and ARBs are primarily initiated and prescribed in the community.
Direct comparison
The head-to-head ONTARGET trial19 comparing an ACEI versus an ARB, demonstrated that the ARB telmisartan was not inferior to the ACEI ramipril for reducing the risk of major CV events overall, including CV death,19 and found fewer side effects in the ARB arm of the trial (mainly due to a reduction in cough).
Another meta-analysis by Volpe et al,21 published in 2009, compared ARBs with other medications that lower blood pressure, including beta-blockers, diuretics, calcium channel blockers and ACEIs as well as placebo. This meta-analysis analysed patients with hypertension, high CV risk, stroke, coronary disease, renal disease, and heart failure, and showed no difference in risk of MI with ARBs compared to the other treatments, but also no significant difference compared to placebo. The blood pressure lowering arm of the ASCO trial also demonstrated that beta-blockers and diuretics are less efficacious in reducing CV risk when compared to ACEIs and calcium channel blockers.22 Volpe et al22 concluded: ‘The present meta-analysis indicates that the risk of MI is comparable with use of ARBs and other antihypertensive drugs in a wide range of clinical conditions’.21
In addition, no reduction in the risk of MI with ARBs compared to placebo was found. This may be consistent with the results of the TRANSCEND study,23 which evaluated the effect of an ARB in high risk patients intolerant of ACEIs and showed no significant reduction in CV events. Conversely, the HOPE study,9 which utilised an ACEI in a similar study design, did show a reduction in CV events, although the background population demographics and co-prescribed medications did differ between the two trials. Neither the meta-analysis by Volpe et al nor the ONTARGET study showed a reduction in MI when ARBs and ACEIs were combined. On the basis of these results, the combination of ARBs and ACEIs is no longer recommended for reduction of CV risk in patients with significantly increased CV risk.
Specific populations – diabetic kidney disease
Different considerations come into play when considering type 2 diabetes and kidney disease. While the evidence base for the prevention of new onset albuminuria is stronger for ACEIs,24–26 the prevention of kidney failure in patients with established nephropathy has been demonstrated in trials using ARBs.27,28 Both classes may therefore have a role individually, but it may be prudent to favour the class or the specific drug that has been demonstrated to be beneficial for particular patient subgroups. There are no head-to-head comparison trials between ACEIs and ARBs in diabetic patients with established kidney disease. Clinicians have to weigh up the risk of a patient dying from a CV event (where ACEIs have consistent clinical evidence of mortality benefit)6 versus the risk of heading toward end stage renal failure requiring dialysis and/or transplantation (where two ARBs have shown clinical benefit against amlodipine28 or non-ACEI antihypertensive agents).27 Renal impairment is an independent risk factor for CV disease.29 While caution is required in employing drugs that manipulate the RAS, there are clear benefits to be obtained from ACEI therapy, even in patients with mild renal impairment.9
What do the guidelines say?
Different clinical guidelines make different recommendations,1,13,29–31 making it confusing to determine the optimal treatment for individual patients. Hypertension guidelines may be at odds with other CV risk factor guidelines, including cholesterol lowering, diabetic guidelines, or other non-CV strategies to reduce risk.1,29–31
ACEIs and ARBs are bundled together in some guidelines, as though these drug classes are interchangeable.1,31 This is not based on evidence, as the trials on which the guidelines are based often utilise only one class of drug. Extrapolation to all drug classes that impact on the RAS may not be clinically appropriate.
The Australian National Vascular Disease Prevention Alliance has recently published the Guidelines for the assessment and management of absolute CV risk,32 which is an excellent resource for general practitioners in this context.
Practical challenges
The evidence of a possible survival advantage of ACEIs over ARBs6,18 is often overshadowed by concern over the risk of cough. The incidence of ACEI induced cough has been reported to be 5–35% among patients receiving treatment with these agents, however a much lower incidence (up to 3%) has been described in studies where the cause of the chronic cough in patients receiving ACEIs has been evaluated.33 Following simple initial screening, the tolerability and adherence to ACEIs is comparable to ARBs in large populations.26,34
Conclusion
Although ACEIs and ARBs are equally effective in lowering blood pressure,5 ACEIs have more consistent data of mortality benefit in the setting of prior MI, coronary artery disease or heart failure.6,18 ACEIs (alone or in combination with a diuretic) have the most direct evidence of benefit in preventing recurrent stroke.35 Selecting a class for renal protection in patients with type 2 diabetes is more complex. Therefore it may be prudent to favour the class, and perhaps the specific drug, that has demonstrated benefit in particular patient subgroups, with ACEIs being initially utilised for CV risk reduction and ARBs being used initially for the prevention and treatment of diabetic nephropathy. Renal impairment is an independent risk factor for CV disease29 and even patients with mild renal impairment can benefit from ACEI therapy.9 ARBs also have a role in patients who cannot tolerate ACEIs because of cough or angioedema.
ACEIs and ARBs are distinct classes that are not interchangeable, and their prescription should be based on clinical evidence. Disparities in the prescription of ACEIs and ARBs require evaluation, discussion and reflection on whether we are doing the best by our patients in the light of the evidence.
Key points
- High blood pressure is the gateway to a cascade of CV risk.
- The aim of treatment in the long term should be to reduce absolute CV risk.
- ACEIs and ARBs are not interchangeable; they are structurally and functionally different.
- Clinical trial data are more consistent for ACEIs than ARBs, particularly for mortality benefit.
- ACEIs should be the preferred agent in the setting of previous MI, coronary artery disease or heart failure because of documented mortality reduction.
Competing interests: Professor Sindone has received honoraria, speaker fees, consultancy fees, is a member of advisory boards or has appeared on expert panels for Abbott, Alphapharm, Aspen, Astra Zeneca, Bayer, Biotronic, Boehringer Ingleheim, Bristol Myer Squibb, Cube, CSL, Elixir, General Electric, GlaxoSmithKline, Guidant, Heart Foundation of Australia, Janssen-Cilag, Johnson and Johnson, Medimark, Medtronic, Merck Sharp & Dohme, Novartis, NSW Department of Health, Ogilvy, Pfizer, Phillips, Roche, Sanofi, Schering Plough, Servier, Solvay, St. Jude, Sunshine Heart, Ventracor and Vifor.
Dr Erlich has received speaker fees, travel support or has been a member of an advisory board for Amgen, Sanofi-Aventis, Servier and Solvay.
Professor Perkovic has received honoraria, speaker fees and consultancy fees, and is a member of advisory boards or has appeared on expert panels for Abbott, Astellas, Astra Zeneca, Boehringer Ingelheim, Heart Foundation of Australia, Johnson and Johnson, Roche, Servier and Vitae.
Professor Suranyi has given talks and presentations on behalf off, received honoraria and travel grants from, and participated in financially sponsored multicentre clinical research funded by Astra Zeneca, Boehringer Ingelheim, Pfizer, Servier and other pharmaceutical companies operating in Australia.
Dr Newman has received honoraria, speaker fees and travelling grants from Astra Zeneca, Bristol Myer Squibb, Novartis, Pfizer, Roche, Sanofi, Servier and Solvay.
Dr Lee has received an honorarium and sponsorship to cardiology meetings from Servier.
Professor Roger has received honoraria, speaker fees, consultancy fees is a member of advisory boards or has appeared on expert panels for Abbott, Amgen, Aspen, Astra Zeneca, Baxter, Bayer, Fresenius Medical, Hoffman-La Roche, Janssen-Cilag, Medimark, Merck Sharp & Dohme, Novartis, Sandoz, Sanofi-Aventis, Servier, Solvay, Takeda and Vifor.
Dr Barin has served as invited speaker for Abbott, Astra Zeneca, Boehringer Ingelheim, Pfizer and Servier on cardiovascular topics.
Dr Shalaby has received honoraria and sponsorship to cardiology meetings from Boehringer Ingelheim, Sanofi Aventis and Servier.
Provenance and peer review: Not commissioned; externally peer reviewed.
Acknowledgement
The authors would like to acknowledge the contribution of Dr Grant Shalaby to the development of this document.