Difficult decision: Office or ambulatory BP measurement
Although hypertension causes cardiovascular disease, clinic blood pressures poorly predict cardiovascular outcomes for individual patients.1 As O’Brien noted, ‘Blood pressure measurement is one of the few areas of medical practice where patients in the twenty-first century are assessed almost universally using a methodology developed in the nineteenth’.2
Imprecision of clinic BP as a predictor of cardiovascular risk is hardly surprising – non-invasive measurement of brachial artery BP is distorted by obesity,3 arterial wall rigidity,4 and partial or complete proximal arterial occlusions – not uncommon in the population that most benefits from cardiovascular risk reduction. Even when accurate, clinic measurements are a ‘snap-shot’ that is shaped by the patient-doctor interaction, and which are blind to diurnal changes in BP and heart rate.5
Ambulatory blood pressure monitoring (ABPM) is a superior predictor of cardiovascular risk.1 However, its legitimacy is potentially limited by an absence of data confirming that treatment decisions based on ambulatory pressure produce superior cardiovascular outcomes. Nevertheless, we have long adopted the concept of managing total cardiovascular risk in preference to treating individual risk factors. Following that logic, it seems sensible to base BP treatment on the measurement that best predicts cardiovascular outcomes.
Authorities such as the United Kingdom’s National Institute for Health and Clinical Excellence, recommend ambulatory monitoring for all patients with clinic pressures >140/90 mmHg.6 Although not as inclusive, Heart Foundation recommendations (Table 1)7 will capture vast numbers of patients attending primary care. Ambulatory monitoring is widely available from Australian pathology vendors and some specialist clinics, but it does not attract a Medicare rebate, and therefore represents a significant expense for many patients.
Table 1. Heart Foundation recommendations for consideration of ambulatory blood pressure monitoring
- To exclude ‘white coat’ hypertension in patients with newly discovered hypertension with no evidence of end-organ damage
- In patients with borderline or labile hypertension
- To assist BP management in patients whose BP is apparently poorly controlled, despite using appropriate antihypertensive therapy
- In patients with worsening end-organ damage, despite adequate BP control on office BP measurements
- To assess adequacy of BP control over 24 hours in patients at particularly high risk of cardiovascular events, in whom rigorous control of BP is essential
(eg. diabetes, past stroke)
- In deciding on treatment for elderly patients with hypertension
- In patients with suspected syncope or orthostatic hypotension
- In patients with symptoms or evidence of episodic hypertension
- Hypertension in pregnancy
|
Use of ABPM in primary care patients detects undiagnosed, over-treated and under-treated hypertension.8 Moreover, international experience suggests the expense of ambulatory monitoring can be offset by savings in drug costs and visits to the doctor.9 Predictably, calls have been made for general practitioners to comply with guidelines.2 For some practitioners, this may represent a substantial change from current practice.
Difficult decision: Who benefits from ambulatory monitoring?
Like any diagnostic technique, ambulatory monitoring is most beneficial when it has the potential to change management.
Ambulatory BP monitoring in borderline or mild hypertension
Ambulatory monitoring can detect white coat hypertension, which is when clinic pressures are elevated but ambulatory pressures are normal. White coat hypertension is not completely benign – affected individuals have a 40% chance of developing hypertension over the next 10 years,10 and they are at higher risk of impaired fasting glucose or diabetes.11 Patients with a diagnosis of white coat hypertension will require confirmation of the diagnosis by repeat ambulatory monitoring within 6 months, continued surveillance by repeating ambulatory monitoring every 1–2 years, and ongoing lifestyle modification.5
Predictors of white coat hypertension include female gender, non-smoking, recent onset or borderline hypertension, and absence of end-organ damage.5 Some of these predictors also signal a relatively low risk of cardiovascular disease. Therefore, the cardiovascular risk of some patients suspected of having white coat hypertension may be low enough to avoid pharmacological therapy, even if ambulatory monitoring detects elevated pressures.
Ambulatory monitoring is thus most likely to influence management if it is applied to patients who have risk factors for white coat hypertension, and in whom cardiovascular risk is high enough for the ambulatory monitor result to change treatment decisions. An example might be an older patient with borderline hypertension and who has cardiovascular risk factors, such as dyslipidaemia or a positive family history of cardiovascular disease.
Conversely, patients with mildly elevated pressure and pre-existing coronary or cerebrovascular disease have little prospect of avoiding pharmacotherapy. Similarly, the presence of left ventricular hypertrophy might be seen as an indication that the office measurement is authentically elevated. In these scenarios an ambulatory measurement has less prospect of affecting management.
Ambulatory BP monitoring in treated hypertension
The 3 million Australians treated for hypertension each year12 represent a potentially overwhelming demand for monitoring hypertension control with ambulatory monitoring. However, among these treated hypertensive patients will be some who are more likely to benefit from ambulatory monitoring than others. Strict BP control offers most benefit to patients with the highest cardiovascular risk. Therefore, even when clinic pressures are within the target range, ambulatory monitoring is particularly useful for patients with diabetes or known atherosclerotic disease.2,13
Ambulatory BP monitoring in ‘normotension’
‘Masked hypertension’ is when there is normal clinic measurements but elevated ambulatory pressures.14 Although much less studied than the ‘white coat’ variant, masked hypertension occurs in a similar proportion of the general population, which is about 10%, and appears to have a prognosis that is similar to sustained hypertension.5
It is not yet viable to screen the entire population with ambulatory monitors to detect masked hypertension. Instead, masked hypertension should be suspected in patients with normal office measurement who inexplicably develop pathology that is typically caused by hypertension, for example, unexplained left ventricular hypertrophy or premature atherosclerotic disease in the absence of other cardiovascular risk factors.
Patients who have more extreme cardiovascular risk are another group that benefit from ambulatory monitoring, even if office measurements are in the normal range. For example, it has been argued that all people with type 2 diabetes should have ambulatory monitoring.13
Difficult decision: How to respond to elevated nocturnal pressure
Having ordered a 24 hour ambulatory monitor, it is not uncommon to be surprised with a result that indicates blood pressures at night are higher than during the day (Figure 1). The importance of this ‘non-dipper’ picture is that it may signal underlying postural hypotension15 (a fall in BP of >20/10 mmHg), which may be causing orthostatic intolerance (symptoms related to upright posture and relieved by recumbence).16
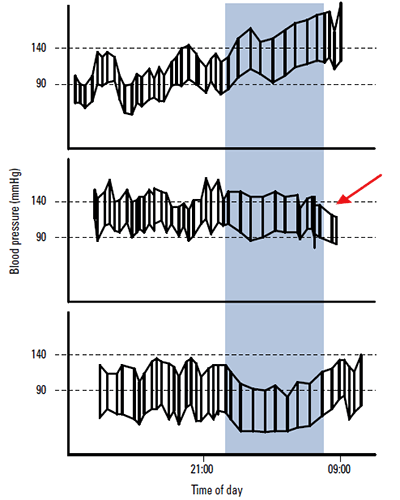
Figure 1. Examples of ambulatory blood pressure patterns
Lower panel: A normal record with a day pressure <135 85="" mmhg="" and="" night="" pressures="" are="" 10="" 20="" lower="" br=""> Middle panel: A ‘non-dipper’ pattern where the pressure falls at night by <10%. the="" arrow="" depicts="" falling="" blood="" pressures="" in="" morning="" which="" were="" associated="" with="" patient="" s="" symptoms="" br=""> Upper panel: ‘Reversed dipping’ in a patient with severe postural symptoms, but whose postural drop was only 18 mmHg
Patients with postural hypotension tend to have lower BP while they are upright (such as during the day), and pressures can increase with recumbence. This can manifest on ambulatory monitoring as blunted or reversed diurnal variation, episodes of hypotension, or excessively variable pressures (Figure 1).
Inadequate BP may also inhibit production of urine during the day.17 Consequently, patients with orthostatic intolerance may complain of day oliguria, nocturia and nocturnal polyuria (which is production of >30% of urine at night). In our experience, abnormal diurnal variation and nocturnal polyuria can indicate orthostatic intolerance, even in the absence of a measured postural BP – which requires patients to be recumbent for at least 3 minutes before standing for the same time.18
Treating postural hypotension begins with falls prevention.19 Patients should be educated about the mechanisms that interfere with maintenance of BP, ie. postural change, prolonged standing, heat, exercise, food and alcohol. Specific fall mitigation strategies should be implemented, such as using a chair for showering and drying, instructing patients to bath in the afternoon, and having a commode or non-spill urine bottle by the bedside. It may also be useful to adjust antihypertensive medications by minimising antihypertensive agents, avoiding diuretics, switching antihypertensive medications to a night dose, and referring the patient to a clinician with expertise in this area.
Difficult decision: Resistant hypertension
Hypertension is resistant when BP exceeds goals, despite using three or more antihypertensive agents from different classes at optimal doses, one of which should be a diuretic. It has also been proposed that the label of ‘resistant hypertension’ should apply to BP that requires four or more medications to control.20
Management of resistant hypertension should always include the steps described in Table 2. However, clinical experience tells us that these measures will only be modestly successful. For some patients this will be because of white coat hypertension, which should be investigated with ambulatory or home measurements.
Table 2. Potential non-pharmacological measures to treat resistant hypertension20
- Address treatment adherence
- instruct patients to bring their medicines to an appointment where the date of medication dispensing can be used to determine if prescriptions are being filled
- measurement of pulse rate may be a surrogate measure of compliance for patients taking beta-blockers
- particularly for elderly patients, non-adherence may be helped by using dosing aids and/or by minimising ‘pill loads’ by employing fixed dose combination products
- Weight loss
- Dietary salt restriction
- urinary sodium excretion can be used to detect excessive dietary sodium intake
- Moderation of alcohol intake
- Increased physical activity
- Ingestion of a high-fibre, low-fat diet
- Where possible, minimise or cease medications that elevate blood pressure
- NSAIDs, glucocorticoids, oral contraceptive pill, sympathomimetics, ephedra, liquorice root
- Detect and treat sleep apnoea
|
After clinic measurements are confirmed, secondary causes of hypertension require consideration. The list of secondary causes is well documented, and includes conditions that activate the renin-angiotensin-aldosterone system (RAS), ie. renal artery stenosis, renal parenchymal disease and primary hyperaldosteronism, and the rare adrenergic cause of a phaeochromocytoma. Therefore, an alternative approach to an exhaustive investigation of secondary causes might be to trial a pharmacological RAS blockade.
Treatment should include maximal doses of a calcium channel blocker, an angiotensin converting enzyme inhibitor (or angiotensin 2 receptor blocker) and a thiazide diuretic. Even though hydrochlorothiazide is Australia’s most popular thiazide, 25 mg of chlorthalidone has superior efficacy – even when compared to 50 mg/day of hydrochlorothiazide.21 At these higher doses, hyponatraemia becomes a significant concern in elderly patients, especially in hotter weather, or during any illness that disturbs fluid or electrolyte homeostasis.
‘Unusual’ medications with demonstrated efficacy in resistant hypertension include spironolactone 25 mg/day, and amiloride 10 mg/day. These drugs reduced BP by 7.3/3.3 mmHg, 12.2/4.8 mmHg and 14.1/5.1 mmHg, when used alone or in combination respectively, in an African-American population.22 Both cause hyperkalaemia and impair renal function, so electrolytes should be frequently measured when initiating therapy. Spironolactone’s other major side effect is anti-androgenism – resulting in nipple pain, gynaecomastia, erectile dysfunction and testicular atrophy. Other unusual medications that may be useful include hydralazine, methyldopa and labetalol.
Chronotherapy is the newest approach to resistant hypertension. Diabetic patients who took at least one of their antihypertensive agents at bedtime had better 24 hour mean BP control and significantly reduced cardiovascular morbidity and mortality.23 Renal artery sympathectomy looks to be a promising alternative, and as longer term data accumulates, it has failed to disappoint.24
Key points
- Ambulatory BP monitoring is of most clinical value when it can change management:
- diagnosing ‘white coat hypertension’ can avert pharmacological therapy if the absolute cardiovascular risk is high enough to justify drug therapy
- ambulatory monitoring may be justified in patients who are at very high cardiovascular risk regardless of their office pressures
- ‘masked hypertension’ should be excluded in patients with unexplained left ventricular hypertrophy or atherosclerosis.
- Postural hypotension can cause elevated night BP and over- production of urine at night.
- Spironolactone or amiloride are useful agents when hypertension remains uncontrolled, despite treatment with at least two full dose antihypertensive medications and a diuretic.
Competing interests: Dr O’Callaghan has received honorarium for educational activities related to antihypertensive medications from Servier Laboratories Australia.
Funding: This work was supported by funding from the Victorian Neurotrauma Initiative.
Provenance and peer review: Commissioned; externally peer reviewed.