Hepatitis C infection is an important chronic disease, affecting 1.4% of the Australian population.1 The primary mode of acquisition is via injecting drug use. As people living with chronic hepatitis C (CHC) infection age, the burden of liver disease, liver failure and hepatocellular carcinoma is expected to increase. Hepatitis C virus (HCV) related liver disease is already the most common indication for liver transplant in Australia. Comorbidities such as alcohol and obesity are important in the progression of fibrosis and should be addressed early.
Hepatitis C is curable, particularly when treated early, thereby preventing the development of cirrhosis and cancer. Recently, the first generation of direct-acting antiviral agents have been approved for the treatment of genotype-1 HCV infection, and when used in combination with pegylated-interferon-alfa (peg-IFN) and ribavirin (RBV), increase cure rates significantly. Treatment will continue to evolve rapidly, with the expected arrival of well tolerated and efficacious all-oral combination therapies (interferon free) by 2015–16 for most genotypes. Thus, all patients should be referred for consideration of treatment.
Incidence and prevalence in Australia
Recent estimates suggest that there are 226 700 people living with the HCV in Australia, of whom 85% have been diagnosed.1–3 Approximately 10 000 new HCV infections occur in Australia per year, with 95% due to injecting drug use. A minority of patients acquire HCV via other modes of transmission; these patients were often born overseas and may have acquired HCV via unsterile vaccination, medical procedures, injection treatment, blood product transfusion from unscreened donors, acupuncture, endoscopy, or cultural practices such as public shaving or cupping.1,2
Despite this prevalence, only 3760 people with chronic HCV infection (less than 2%) are treated each year in Australia. The reasons for poor treatment uptake are multifactorial: relatively low overall treatment efficacy (54–56% for peg-IFN and RBV), long treatment duration (6–12 months), significant physical and psychological side effects, a high rate of comorbidities such as substance abuse and psychiatric disease, poor patient understanding of disease progression, and long waiting times to be assessed at tertiary hospital liver clinics.4–6
Natural history of infection
Acute hepatitis C
Duration of infection less than 6 months
Acute hepatitis C is defined as clinical signs or symptoms of hepatitis or HCV antibody seroconversion within 6 months of presumed exposure. Only 16% of patients are symptomatic and the illness is often missed.7 Symptoms may include fatigue/lethargy, myalgias, low grade fever, nausea and vomiting, right upper quadrant pain and jaundice. Twenty-five percent of patients will clear the infection spontaneously within 6 months of infection, but 75% progress to chronic hepatitis. Jaundice, symptomatic hepatitis, and the presence of the favourable host IL28B genotype, ‘CC’, are associated with spontaneous clearance.8 Antibodies are not protective and patients can be reinfected with the same or other HCV genotypes following re-exposure.
Chronic hepatitis C
Duration of infection more than 6 months
Patients with CHC are usually asymptomatic, but may have non-specific symptoms such as fatigue, nausea and right upper quadrant discomfort. Symptoms do not correlate with disease severity or progression. Chronic hepatitis C is a slowly progressive disease, with approximately 5–7% of community cohorts progressing to cirrhosis at 20 years.9 Factors associated with faster disease progression include significant alcohol ingestion (>40 g/L), co-infection with hepatitis B or HIV, age over 40 years at acquisition, marijuana use and obesity. Women appear to progress more slowly than men. To date, there have been no viral factors associated with progression. The natural history of CHC is presented in Figure 1.9–17
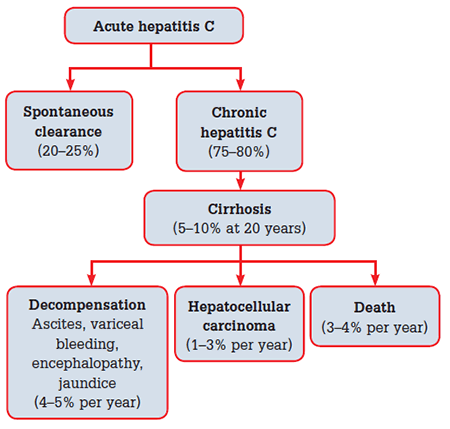
Figure 1. Natural history of hepatitis C virus
In 2006, 5600 individuals were estimated to have HCV related cirrhosis,18 but this number is expected to triple by 2020, resulting in an increase in the number of liver related admissions to hospital and deaths from liver failure and hepatocellular carcinoma.19 Currently HCV is the leading indication for liver transplantation, and hepatocellular carcinoma has the fastest rising incidence of any cancer in Australia, reflecting the increasing burden of end-stage liver disease.20 Modelling studies estimate that treatment numbers need to rise to >10 000 per year to reduce long term disease burden and cost.18
Initial assessment and management
Recommended screening for HCV and initial assessment of the patient is outlined Table 1. Patient education is summarised in Table 2.21,22
Table 1. Recommended screening for hepatitis C virus and initial patient assessment
| Who to screen |
|---|
- Patients with abnormal liver function tests
- History of past or current intravenous (and intranasal) drug use
- Received a blood transfusion or blood products before 1990
- Migrants from high prevalence regions (Egypt, Pakistan, Mediterranean and Eastern Europe, Africa and southern Asia)
- History of tattoos or piercings
- History of incarceration
- Children of mothers with HCV
- Healthcare workers performing exposure prone procedures
|
| What to screen with |
|---|
- HCV antibody (serology): exposure to HCV (past or current infection)
- HCV RNA (qualitative PCR): current active HCV infection
- Liver function tests: raised ALT/AST
|
| Examination findings |
|---|
| Although neither specific or diagnostic, the following examination findings may suggest the underlying presence of cirrhosis |
| Peripheral |
Palmar erythema
Dupuytren contracture
Clubbing
Leukonychia
Peripheral hair loss |
Asterixis
Petechiae or ecchymoses
Muscle wasting
Ankle oedema |
| Face and chest |
Jaundice
Fetor hepaticus
Gynaecomastia |
Parotid enlargement
Spider naevi |
| Abdomen |
Splenomegaly
Ascites
Caput medusae |
Hepatomegaly (only in alcoholic liver disease, haemochromatosis or with hepatocellular carcinoma) |
| Investigations |
|---|
| Recommended tests | Interpretation |
|---|
- Liver function tests
- INR
- Full blood count
- HCV genotype
- Hepatitis A and B and HIV serology
- Liver ultrasound
|
Inflammatory activity: ALT, AST
Synthetic function: albumin, bilirubin, INR
Portal hypertension: platelet count
Determines treatment options
Important co-infections
Hepatitis A and B vaccines indicated if not immune
Excludes focal lesions, significant portal hypertension |
| Optional tests (ordered by specialist) |
|---|
- HCV RNA level
- Host IL28B genotype
- Liver FibroScan® or biochemical marker of liver fibrosis
|
Quantitative PCR – predicts interferon response
Host polymorphism that predicts interferon response and spontaneous clearance
Non-invasive marker of liver fibrosis/cirrhosis |
Table 2. Recommended education for patients with hepatitis C virus
|
Transmission
Blood
|
HCV is a blood borne virus, and hence is transmitted through direct blood-to-blood contact
HCV positive patients are not allowed to donate blood
Toothbrushes and razors should not be shared
|
|
HCV and pregnancy
|
Mother-to-baby transmission is low (5–7%)
Maternal HCV antibodies are present in infants for 18 months, hence testing should be performed after this time
Caesarean section does not reduce the risk of transmission
Breast milk should be discarded if there is bleeding or cracked nipples
|
|
Sexual
|
Sexual transmission is very low among long term monogamous heterosexual partners (0–0.6%/year)
|
|
Injecting drug use
|
HCV positive patients with ongoing intravenous drug use should be referred to needle syringe exchange programs
Past HCV infection does not prevent re-infection
|
|
Alcohol consumption
Patients should be advised to abstain from alcohol if possible. Those who consume more than 40 g/day have an increased risk of more rapidly progression liver disease and should be counselled to stop alcohol consumption, and should be referred to drug and alcohol services
|
|
Vaccination
Currently there is no vaccine for HCV
Patients should be tested for and vaccinated against hepatitis A and hepatitis B if not immune
|
|
Herbal remedies
Silymarin (milk thistle) has been shown not to improve ALT, HCV RNA levels or outcomes in chronic hepatitis C20,21
|
Who and when to refer for assessment and treatment
The availability of efficacious new drugs for HCV, and the promise of well tolerated all-oral therapy on the horizon, means that every patient should now be considered for treatment. It is particularly important that patients with suspected cirrhosis are referred, as these patients have the most to gain from viral eradication, and require surveillance for complications such as varices and hepatocellular carcinoma.
The timing of treatment is determined by the stage of liver disease (mild-moderate vs advanced), by comorbidities that may complicate treatment, and by patient choice. The decision for treatment may be impacted upon in patients with a significant past history or current history of psychiatric illness, particularly those requiring hospitalisation, and those with active poorly controlled substance abuse, as these patients may not be suitable for current HCV therapy. However, these patients should still be referred for assessment, as they may have significant underlying liver disease, or may be suitable for the newer HCV therapies.
Hepatitis C treatment in 2013
Hepatitis C virus infection is curable, and viral eradication will prevent the long term liver complications of HCV infection. For the past decade, the standard-of-care treatment for CHC infection has been dual therapy with pegylated-interferon-alphaαand ribavirin (peg-IFN+RBV). Treatment regimens are rapidly evolving. The first two direct-acting antiviral agents (DAAs) for genotype-1 HCV (HCV-1) were approved in Australia in 2012, and have been Pharmaceutical Benefits Scheme listed from 1 April 2013. These two drugs, telaprevir and boceprevir, are both inhibitors of the HCV NS3 protease, which is necessary for viral replication. They are specific for HCV-1. Both drugs must be used in combination with peg-IFN+RBV to prevent drug resistance. Triple therapy with telaprevir or boceprevir has significantly increased cure rates from 40–50% to 70–75%, and offers the possibility of short duration therapy in approximately 50% of patients. Triple therapy is also effective for patients who have previously failed dual therapy with peg-IFN+RBV. Peg-IFN+RBV remains the standard of care for all other HCV genotypes. Table 3 outlines the current treatment regimens, duration of therapy, expected cure rates (sustained virological response, defined as negative HCV PCR at 6 months post cessation of therapy), potential side effects and their management.
Table 3. Hepatitis C virus treatment regimens and side effects
| HCV treatment regimens |
|---|
| HCV genotype | Treatment regimen | Duration | Sustained virological response rates (cure) |
|---|
| Genotype-1 HCV |
- Telaprevir plus peg-interferon-alpha + ribavirin
- Boceprevir plus peg-interferon-alpha + ribavirin
|
24–48 weeks
28–48 weeks |
70–75% |
| Genotype-2/3 HCV |
- Peg-interferon-alpha + ribavirin
|
24 weeks |
80% |
| Other HCV genotypes |
- Peg-interferon-alpha + ribavirin
|
48 weeks |
40–70% |
| Side effects of HCV therapies |
|---|
| Medication | Major side effects |
|---|
| Peg-interferon-alpha |
Flu-like symptoms, fatigue/lethargy, neuropsychiatric (insomnia, irritability, depression, psychosis), bone marrow suppression (neutropenia, thrombocytopenia) |
| Ribavirin |
Haemolytic anaemia, nausea, rash |
| Telaprevir |
Anaemia (additive to RBV), rash (occasionally life threatening), gastrointestinal upset, anorectal symptoms (burning, pruritus) |
| Boceprevir |
Anaemia (additive to RBV), nausea, dysgeusia (altered metallic taste) |
Unfortunately, current treatment regimens are often poorly tolerated. Not all patients are candidates for therapy, and appropriate patient selection is important. Patients with cirrhosis have a greater risk of treatment related adverse events. Patients with decompensated cirrhosis cannot currently be treated. They should be referred to a tertiary centre for assessment. Treatment is also contraindicated in patients with in some autoimmune disorders, and with significant or unstable psychiatric disease, particularly psychosis and severe depression.
As successful interferon therapy requires good compliance and regular review, isolated or socially unstable patients may struggle to complete treatment. This includes the homeless, recently discharged prisoners and chaotic injecting drug users. Stable individuals with stable drug habits can often be treated with co-operation between their hepatitis C physician and experienced drug and alcohol physician. Pharmacotherapy for substance abuse is often helpful before antiviral therapy, and methadone or buprenorphine should not be weaned before referral.
Fortunately, interferon-free treatment regimens are now on the horizon. Oral DAAs and combinations that target multiple steps in the viral life cycle are in development. These include next generation protease inhibitors, polymerase inhibitors (nucleoside, non-nucleoside) and NS5a inhibitors. All oral combination DAA regimens promise high efficacy rates, short duration and significantly reduced toxicity. It is likely that the first IFN-free regimens will be licensed in Australia within the next 3–5 years.
HCV and HIV co-infection
Hepatitis C virus and HIV share similar modes of transmission, and therefore co-infection with HCV and HIV is common. All HIV-positive patients should be screened for HCV. Chronic liver disease has emerged as one of the most important comorbidities among HIV-HCV co-infected patients, and data suggest that the natural history of HCV is more aggressive in HIV-positive patients. Antiviral therapy should therefore be considered and both treatment regimens and outcomes are similar to those seen in mono-infected patients. However, there are many drug interactions that must be taken into consideration when prescribing DAAs, in particular with the anti-retroviral agents that are used in HIV treatment. These patients should be treated by physicians who are experienced in managing HIV and HIV/HCV co-infection, or be enrolled in shared care programs if treating doctors are less familiar with the newer HCV therapies.
Conclusion
Chronic hepatitis C infection remains a significant health issue and may lead to the development of liver cirrhosis, liver failure and hepatocellular carcinoma. The incidence of these complications is expected to continue to rise and peak by 2020. However, HCV is curable, and viral eradication has been shown to reduce these complications and improve quality of life. Hepatitis C virus remains underdiagnosed; hence, all patients with risk factors or a high clinical index of suspicion should be screened and referred for treatment. The addition of telaprevir and boceprevir for genotype-1 infection, which have recently been approved in Australia, has significantly increased cure rates and will hopefully improve treatment uptake. The HCV treatment landscape is rapidly evolving, with highly efficacious interferon-free all-oral therapies on the horizon.
Competing interests: Alexander Thompson has received payment as an Advisory Board member for Abbvie, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Novartis and Roche; speaker for Gilead, Janssen, Merck Sharp & Dohme and Bristol-Myers Squibb; research grants from Gilead, Merck Sharp & Dohme and Roche. Sally Bell has received payment as a speaker for Merck Sharp & Dohme.
Provenance and peer review: Commissioned; externally peer reviewed.