The major determinants of obesity are multifaceted, surprisingly poorly understood and extend well beyond simplistic explanations about high energy Western diets and obligatory reductions in human movement.5 The interplay between humans and the environment, influenced by genes, epigenetic default metabolic programming, the intrauterine environment and early infant feeding practices set the scene in the early years for weight trajectory throughout life.6 The list of comorbidities associated with both excess weight and the metabolic consequences of obesity is extensive and encompasses chronic disease, as well as functional and psychosocial disability (Table 1).7 Furthermore, it is well established that increasing levels of obesity are associated with poor overall quality of life and increased morbidity and mortality.8
Table 1. Health risks associated with overweight and obesity in adults
| Body system | Health risk |
|---|
| Cardiovascular |
Stroke
Coronary heart disease
Cardiac failure
Hypertension |
| Endocrine |
Type 2 diabetes
Polycystic ovary syndrome |
| Gastrointestinal |
Non-alcoholic fatty liver disease
Gallbladder disease
Pancreatic disease
Gastro-oesophageal reflux disease
Cancers of the bowel, oesophagus, gall bladder and pancreas |
| Genitourinary |
Chronic kidney disease – glomerulopathy End-stage renal disease
Kidney cancer
Kidney stones
Prostate cancer
Stress urinary incontinence (women)
Sexual dysfunction (men) |
| Pulmonary |
Obstructive sleep apnoea
Obesity hypoventilation syndrome
Asthma |
| Musculoskeletal |
Osteoarthritis – especially the knees
Spinal disc disorders
Lower back pain
Disorders of soft tissue structures such as tendons, fascia and cartilage
Foot pain
Mobility disability (particularly in older adults) |
| Reproductive health |
Menstrual disorders
Miscarriage and poor pregnancy outcome
Infertility/sub-fertility
Breast cancer (postmenopausal women)
Endometrial cancer
Ovarian cancer |
| Mental health |
Depression
Eating disorders – binge eating disorder
Reduced health – related quality of life |
| Adapted with permission from National Health and Medical Research Council. Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia. Canberra: NHMRC, 2013 |
Prevention of obesity is failing for many reasons including: a poor understanding of the obesity determinants and evidence regarding what influences them, political inertia associated with a modern market economy, and philosophical views of personal responsibility versus regulation and whole of community involvement. Treatment strategies for obesity should ideally follow a chronic disease model of care with a patient centred focus and initial use of lifestyle and micro-environmental interventions, with escalation to more intensive interventions as dictated by the severity of disease and response to therapy.9,10 Treatment needs to be evidence based and focused on a broad range of health outcomes, not simply on weight. Excellent management of medical, psychological and physical co-morbidity are critical to engaging patients in weight loss interventions, improving function and quality-of-life, and reducing morbidity and mortality. It is also important to note that not all methods to treat obesity are equally effective. This article addresses first-line treatment with lifestyle modification in general practice and then focusses on appropriate use of more intensive treatments to support weight loss as well as identifying indications for referral to specialist weight management clinics.
Weight management in general practice
General practitioners are often the first healthcare providers to identify overweight or obesity. Treatment should be individualised with careful consideration given to the severity of the problem and associated complications using the 5As approach for weight management: Ask and Assess, Advise, Assist and Arrange (Table 2).7
Table 2. The 5 As overweight and obesity management model for adults and post-pubertal adolescents7
|
Establish a therapeutic relationship, communicate and provide care in a way that is person centred, culturally sensitive, non-directive and non-judgemental
|
| Ask and Assess |
Standard care | Active management |
| BMI <25 | BMI 25–29.9 | BMI 30–34.9 | BMI 35–39.9 | BMI >40 |
|---|
| Routinely assess and monitor BMI and waist circumference (WC) |
Routinely assess and monitor BMI and WC
Discuss if BMI and/or WC increasing
Screen for and manage comorbidities |
Routinely assess and monitor BMI and WC
Discuss health issues
Screen for and manage comorbidities
Assess other factors related to health risk
Blood pressure, lipid profile, fasting glucose, liver function tests, and ask about symptoms of sleep apnoea and depression |
| Advise |
Promote benefits of healthy lifestyle
Explain benefits of prevention of weight gain and maintenance of healthy weight |
Promote benefits of healthy lifestyle
Explain benefits of weight management |
| Assist |
|
Assist in setting up weight loss program:
- Advise lifestyle interventions
- Based on comorbidities, risk factors and weight history, consider adding intensive weight loss interventions (eg. VLEDs, pharmacotherapy, bariatric surgery)
- Tailor the approach to the individual
- Refer to multidisciplinary team for specialist treatment recommendations. Suitable patients include those with severe complex obesity for example those with a BMI >40, BMI >35 with any serious comorbidity, and those BMI 30–35 with serious comorbidity and a positive weight trajectory.
|
| Arrange |
|
Review and monitoring
Long term weight management
Referral to specialist weight management clinic if indicated |
It is important to assess the level of obesity by BMI, distribution of weight (waist circumference), and the extent of co-morbidity, in order to provide effective treatment and assess level of disease risk (Table 3).7,11,12 Patient engagement as a central agent in management is fundamental. The therapeutic partnership is critical in delivering long term health outcomes as for any other chronic disease.9
Table 3. Classification of disease risks* by WHO BMI classification and WC thresholds
| BMI (kg/m2) | Classification | Men WC 94–102 cm
Women WC 80–88 cm | Men WC >102 cm
Women WC >88 cm |
|---|
|
18.5–24.9
|
Normal weight†
|
–
|
–
|
|
25–29.9
|
Overweight
|
Increased
|
High
|
|
30–34.9
|
Obese class I
|
High
|
Very high
|
|
35–39.9
|
Obese class II
|
Very high
|
Very high
|
|
≥40.0
|
Obese class III
|
Extremely high
|
Extremely high
|
|
* Disease risk for type 2 diabetes, hypertension and cardiovascular disease
† Increased WC can also be a marker for increased risk even in persons of normal weight
Reproduced from the Scottish Intercollegiate Guidelines Network (SIGN). Management of obesity. A national clinical guideline. Edinburgh: SIGN; Year. (SIGN publication no. 115). [cited 10 July 2013]. Available from URL: www.sign.ac.uk
|
Optimal management of obesity in time poor general practice requires a team care approach involving those specifically trained and experienced in obesity management. These may include dieticians, practice nurses, commercial weight management programs, exercise physiologists and psychologists.7 General practitioners are encouraged to identify, engage and regularly communicate with local weight management providers and to refer those with resistant severe complex obesity for specialised assessment and management recommendations.7 The evidence demonstrating the benefits of weight loss is well documented. Modest weight loss of 5–10% of starting weight can result in significant health benefits, with substantial weight loss offering even greater improvements in obesity related comorbidities. Weight loss for most isn’t easy. Regulation of body weight is carefully controlled by a range of highly efficient homeostatic mechanisms that work to prevent weight loss rather than to protect against weight gain.13–15 In addition, factors predisposing an obese patient to weight gain – such as certain medications, smoking status, a patient’s weight history and readiness to change – can significantly impact on weight loss success.7 These factors and mechanisms challenge successful weight loss and long term weight maintenance for the obese patient and should be taken into careful consideration, especially when planning interventions.
Despite these difficulties, lifestyle interventions remain the first line treatment for overweight and obesity. General practitioners should make patients aware of the health risks associated with increases in BMI and the benefits that can be derived from lifestyle change, even when independent of weight loss.7 The initial approach to weight loss and lifestyle change should include an emphasis on healthy eating with a subsequent reduction in energy intake, in line with the Australian Dietary Guidelines 2013.16 Increasing levels of physical activity and reductions in sedentary behaviour should also be encouraged.7,12,16,17 Psychological therapies to support behaviour change may also be of assistance.
Intensive interventions
Intensive interventions to potentiate weight loss may involve use of very low energy diets (VLEDs), pharmacotherapy and bariatric surgery. A summary of the weight loss effects of each weight management intervention is shown in Figure 1.
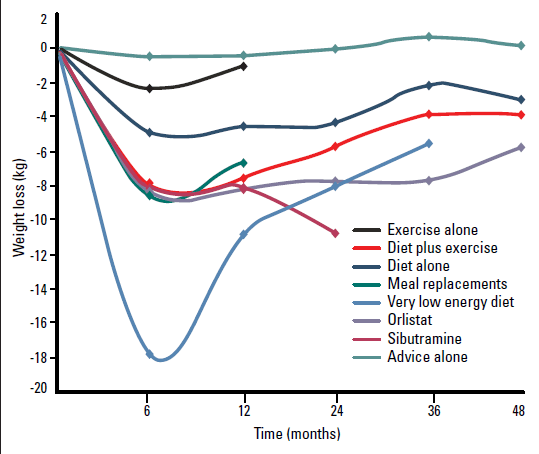
Figure 1. Average weight loss of subjects completing a minimum 1 year weight management intervention; based on review of 80 studies (N=26 455; 18 199 completers [69%])26
Very low energy diets
VLEDs (<800 kcal/day or <3350 kJ/day] are indicated for use in patients with a BMI >30 or BMI >27 with obesity related comorbidities. When used under the medical supervision of a GP and dietician, VLEDs are able to induce rapid weight loss and have been shown to achieve an average weight loss of 18–20% with better sustained weight reduction.18 In addition to weight loss effects, the rapid weight loss offered by VLEDs has been shown to improve glycaemic control in patients with type 2 diabetes, improve blood pressure and reduce total cholesterol. VLEDs involve replacing all meals with a specific meal replacement formula (additional food can be carefully added) during the intensive early phase. These high protein-low carbohydrate diets induce fat burning and mild ketosis, which results in suppression of hunger and promotion of satiety. Treatment duration with a VLED is generally 8–12 weeks, however, safe year-long use under strict medical supervision has been reported.19 In addition, VLEDs are safe and effective when used to assist with long term weight maintenance in either an intermittent or on-demand fashion.20
VLEDs may not be suitable for use for all obese patients and it is important to consider the costs associated with purchasing suitable nutritionally complete meal replacements. VLEDs are contraindicated for use in pregnant or lactating women, infants, children, adolescents (under 18 years), elderly (over 65 years), patients with a history of psychological disturbances, alcohol misuse or drug abuse, in the presence of porphyria, recent myocardial infarction or unstable angina.7 Monitoring and support of patients on VLEDs is required for success (Table 4). Training on the use of VLEDs is available and should be sought by practices wanting to effectively utilise this intensive intervention with suitable overweight or obese patients.
Table 4. VLEDs: Adverse effects, monitoring and review in general practice7,19
| Adverse effects |
Common |
Sensitivity to cold, dry skin, temporary rash, temporary hair loss, postural hypotension, dizziness, fatigue, diarrhoea, constipation, muscle cramps, bad breath, irritability, menstrual disturbances |
| Rare and serious |
Gallstones, gout, sodium or potassium imbalance, temporary changes in liver enzyme levels, reduced bone mineral density |
| Monitoring |
Medical |
Medical history, physical examination, height, weight, waist and hip circumference, BMI, blood pressure, pulse, electrocardiogram |
| Blood biochemistry* |
Full blood count, iron studies, electrolytes, creatinine, uric acid, liver function tests, lipid profile and urinalysis including ketones, pH and microalbuminuria |
| Medications |
Anti-diabetic agents (sulphonylureas, thiazolidinediones, insulin), warfarin, lithium, diuretics, anti-psychotics, anti-convulsants (valproate, gabapentin, carbamazepine) |
| Review frequency |
GP |
Each week for 4 weeks, then fortnightly for remainder of intensive phase |
| Dietician |
| * Tests carried out on patients should be completed at the beginning of a VLED. An ECG is only needed if there is another indication. Blood biochemistry may be repeated at 6 weeks if indicated by the patients accompanying medical condition and for all after 3 months if they they intend to continue with the intense VLED program. |
Pharmacotherapy
Pharmacotherapy for the treatment of obesity should be considered for use as an adjunct to lifestyle intervention in patients with a BMI >30 or BMI >27 with obesity related comorbidities.21 Weight loss medications used in the treatment of obesity can act centrally to increase levels of satiety or act on the gastrointestinal tract to restrict nutrient absorption. Table 5 describes the pharmacological agents that may be used to treat obesity.7,17 Care, consideration and close monitoring is essential when prescribing these medications. The United States Food and Drug Administration (FDA) has recently approved two new medications: lorcaserin and phentermine-topiramate.
Table 5. Weight loss and other medications for treatment of obesity
| Action | Medication | Indications | Common adverse effects | Mean weight loss (compared with placebo) | Notes |
|---|
| Dopaminergic agonist |
Phentermine |
Management of obesity as a short term adjunct to medically managed comprehensive weight reduction regimen in obesity of BMI >30 or BMI 25–29.9 if associated with comorbidities |
Palpitations, tachycardia, hypertension, precordial pain, central nervous system stimulation, headache, gastrointestinal upset including constipation, dry mouth, altered taste, micturition disturbance, rash, impotence, libido change, facial oedema |
3.6 kg (CI: 6.0–0.6) following 2–24 weeks treatment26 |
Approved only for short term use – up to 3 months |
| Pancreatic and gastric lipase inhibitor |
Orlistat |
Treatment of obese patients with a BMI >30 and overweight patients with a BMI >27 with associated comorbidities |
Gastrointestinal upset including oily spotting, flatulence, faecal urgency, loose stools, nausea, dyspepsia, reduced vitamin absorption, headache, kidney stones |
2.9 kg (CI: 3.5–2.3) at
1 year27 |
Low fat diet should be followed |
| Biguanide |
Metformin |
Treatment of type 2 diabetes |
Gastrointestinal upset, taste disturbance, vitamin B12 depletion, liver function test abnormality, hepatitis, skin reaction |
Women of reproductive age prone to infertility:
0.68 BMI (CI: 1.13–0.24) following 35 days to 6 months treatment28
Without diabetes treated with atypical anti-psychotics:
4.8% body weight
(CI: 8.0–1.6) following 12–14 weeks treatment29 |
Not approved for the treatment of obesity |
| Glucagon-like peptide agonists |
Exenatide (Byetta) |
Treatment of type 2 diabetes |
Gastrointestinal upset, hypoglycaemia, exenatide antibody formation, decreased appetite, headache, hyperhidrosis, jitteriness, asthenia, injection site reaction, nasopharyngitis, upper respiratory tract infection, back pain, cough |
Without diabetes:
3.2 kg (CI: 4.3–2.1) following minimum 20 weeks treatment30
With diabetes:
2.8 kg (CI: 3.4–2.3) following minimum 20 weeks treatment31 |
Byetta and Victoza are not yet approved for the treatment of obesity |
| Liraglutide |
Treatment of type 2 diabetes |
Gastrointestinal upset, anorexia, dyspepsia, eructation, gastroesophageal reflux disease, hypoglycaemia, decreased appetite, headache, injection site reaction, upper respiratory tract infection, antibody formation, urticaria, oedema, pancreatitis, thyroid neoplasm, goitre, increased blood calcitonin, renal failure |
| Serotonin 2C receptor agonist |
Lorcaserin |
Management of obesity as an adjunct to medically managed comprehensive weight reduction regimen in obesity of BMI >30 or in overweight of BMI >27 if associated with comorbidities |
In non-diabetic patient: headaches, dizziness, fatigue, nausea, dry mouth, constipation
In diabetic patient: hypoglycaemia, headache, back pain, cough, fatigue |
3.23 kg (CI: 3.75–2.70) at
1 year32 |
Lorcaserin and Qsymia are both FDA approved, but not yet approved by the TGA for use in Australia |
| Combined dopaminergic agonist and antiepileptic |
Phentermine and topiramate |
Management of obesity as an adjunct to medically managed comprehensive weight reduction regimen in obesity of BMI >30 or in overweight of BMI >27 if associated with comorbidities |
Paresthesia in the hands, arms, feet, or face, dizziness, dysgeusia, insomnia, constipation, dry mouth, tachycardia, suicidal thoughts |
7.5 mg phentermine plus 46.0 mg topiramate:
8.1 kg (CI: 8.5–7.1) following 56 weeks treatment33
15 mg phentermine plus 92 mg topiramate:
10.2 kg (CI: 10.4–9.3) following 56 weeks treatment33 |
These medicines are not yet approved for use by the Therapeutic Goods Administration (TGA) in Australia.21 It is important to note that the safety and efficacy of co-administration of lorcaserin or phentermine-topiramate with other products for weight loss, and the effects of these medications on cardiovascular morbidity and mortality, have not yet been established.
Surgery
Bariatric surgery should be considered for patients with a BMI >40 or with a BMI >35 with obesity related comorbidities.22 Bariatric surgery is the most effective available treatment for obesity in terms of achieving and maintaining substantial weight loss long term.23 The three most commonly performed procedures in Australia include laparoscopic adjustable gastric banding (LAGB), Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). We are now starting to learn about how alterations to the gastrointestinal tract, induced by bariatric surgery, reduce hunger, increase satiety and confer other metabolic benefits as well as sustained weight loss.24,25
To date, the long term safety of LAGB and RYGB has been documented, however evidence on long term safety is lacking for the SG. Each procedure is accompanied by its own advantages and disadvantages, and these need to be taken into consideration when assessing a patient’s suitability for surgery (Table 6). Current medical and psychological comorbidities, as well as ability to provide informed consent, will all influence a patient’s suitability for undergoing a particular procedure.7 Patients considering bariatric surgery should be made aware of the commitment to indefinite post-surgical care and long term monitoring from an experienced team.
Table 6. Bariatric surgery: A summary of the characteristics of current conventional procedures24
| Surgical procedure | Description | Excess weight loss at 3–5 years* | Percentage mean weight loss | Pattern of weight loss | Morbidity at 1 year | Nutritional concerns | Follow up requirements | Advantages | Disadvantages |
|---|
| Laparoscopic adjustable gastric banding (LAGB) |
Involves placing an adjustable band around the gastroesophageal junction, thereby restricting food intake. The band can be tightened and loosened over time to alter the extent of restriction |
54% |
20–30% |
Gradual; usually maximal at 2–3 years |
4.6% |
Low (deficiencies in iron, vitamin B12, folate) |
Lifelong (assessment and nutritional support), frequent in the first 12 months |
Effective, with good long term weight maintenance
Ability to adjust the degree of restriction
Reversible
Maintains gastric integrity |
Gastric pouch dilatation, erosion of band into the stomach, leaks to the LAGB system, weight regain |
| Roux-en-Y gastric bypass |
Is a combination procedure in which a small stomach pouch is created to restrict food intake and the lower stomach, duodenum and first portion of the jejunum are bypassed to produce modest malabsorption of nutrients and energy intake |
60%
(75% with banded RYGB) |
25–35% |
Rapid; maximal at 1–2 years |
14.9% |
Moderate (deficiencies in iron, vitamin B12, folate, calcium, vitamin D, copper, zinc) |
Lifelong (assessment and nutritional support) |
Very effective with good long term weight maintenance
Few failures |
Abdominal pain, staple line leak, stomach ulcer, intestinal obstruction, gallstones, nutritional deficiency, weight regain |
| Sleeve gastrectomy |
Involves removing the greater portion of the fundus and body of the stomach, reducing its volume from about 2.5 L to about 250 mL |
50–60%
(limited reports at ≥3 years) |
20–30% |
Rapid; maximal at 1–2 years |
10.8% |
Moderate (deficiencies in iron, vitamin B12, folate, calcium, vitamin D, copper, zinc, thiamine) |
Lifelong (assessment and nutritional support) |
Allows for rapid weight loss
No dumping syndrome as pyloric portion of the stomach is in tact
Provides fixed restriction and does not require adjustment |
Staple line leak, gastroesophageal reflux disease, dilatation of the gastric remnant, weight regain |
Contraindications for bariatric surgery: current drug or alcohol abuse; uncontrolled psychiatric illness and lack of comprehension of the risks and benefits, expected outcomes, alternatives and lifestyle changes required with bariatric surgery; the presence or suspicion of esophageal or gastric malignancy; portal hypertension with varices; liver failure; Crohn disease; recently diagnosed malignancy; multiple organ failure; previous gastric or gastrointestinal surgery; recent myocardial infarction (may substantially increase the risk of surgery, later complications or poor outcomes)
* Excess weight defined as the weight of an individual in excess of their weight at BMI 25 kg/m2 |
Specialist weight management clinics
Unfortunately, specialist weight assessment and management clinics for complex severe obesity are not broadly available, but with the emergence of new drugs, devices and surgical procedures, as well as ever increasing patient numbers; assessment by teams skilled in this area is becoming more necessary. Some major hospitals offer outpatient ‘specialist weight management’ or ‘metabolic’ clinics; however, access is often impeded by very long waiting lists. Medicare Locals may provide a forum for exploring delivery gaps in regional areas particularly. Specialised weight management services would provide advice to the GP similar to that expected from cardiac or diabetes referrals such as an evaluation of the patients, advice regarding the treatment options and a proposal for ongoing shared care. Severe obesity is a serious complex chronic disease and requires this level of expertise and support to optimise health outcomes.
Conclusion
General practitioners are in a key position to provide support, advocacy and coordinate management for obese patients. The use of intensive interventions should be considered and utilised within the general practice setting and, where indicated, complex obese patients should be referred to specialist weight assessment and management clinics.
Key points
- Obesity is a complex chronic relapsing condition requiring ongoing management and monitoring from medical and other allied health professionals.
- Maintained weight loss is a primary, but not the sole, consideration when treating obesity.
- General practitioners are encouraged to consider the timely use of more intensive treatments to support weight loss beyond that of first line treatment with lifestyle modification.
- Referral and liaising with specialist weight assessment and management clinics for complex cases can enhance outcomes.
Competing interests: John B Dixon is a board member of Nestle Australia and has received payment for consultancy from Allergan Inc and Bariatric Advantage. John B Dixon has received payments for lectures from iNova Pharmaceuticals and Merck Sharp & Dohme and for development of educational presentations from iNova Pharmaceuticals, and travel expenses from GI Dynamics.
Provenance and peer review: Commissioned; externally peer reviewed.