How does it work?
Myocardial perfusion scans (MPS) are obtained after the intravenous (IV) injection of a small dose of radiotracer at rest and following physiological (exercise) or pharmacologic (adenosine, dipyridamole or dobutamine) stress. Originally, thallium was used but it has been superseded by the technetium-labelled synthetic compounds, sestamibi and tetrofosmin, which yield better quality images at a lower radiation dose.
As in other nuclear medicine procedures, the radiotracer emits photons, which are detected by a gamma camera. Newer cameras are equipped with X-ray computed tomography (CT) scanners, which permit compensation for the differential absorption of photons through the complex and variable anatomy (soft tissue, lung and bone) of the chest (attenuation correction (AC)).
The best physiologic stressor for MPS is exercise using a standardised treadmill or bicycle ergometer protocol; exercise capacity is an independent prognostic variable in patients with CAD. However, because of age, comorbidities or concurrent medications, many patients are unable to exercise sufficiently to achieve an adequate cardiac stress (more than 85% of the age-predicted maximum heart rate and five metabolic equivalents (METs)), and therefore undergo pharmacological stress. The most common pharmacologic agents used in MPS are the coronary vasodilators, dipyridamole and adenosine. Dipyridamole and adenosine MPS have been shown to have equivalent sensitivity and specificity as exercise MPS for the diagnosis of CAD.2
After scanning, the tomographic data are processed and reconstructed into a conventional format (Figure 1a). Since regional tracer uptake is proportional to perfusion, areas with reduced perfusion, such as ischaemic segments, will appear as areas of less intense or absent tracer uptake (Figure 1b). The individual patient’s perfusion map can be compared to a gender-matched reference database for a more objective, semi-quantitative assessment of the extent and severity of perfusion abnormalities. When the data are captured in synchrony with the cardiac cycle (electrocardiogram (ECG) gating), regional and global left-ventricular systolic function can also be assessed (Figure 2). The end-diastolic and -systolic volumes (EDV, ESV) as well as ejection fraction (EF) can be calculated from this analysis.
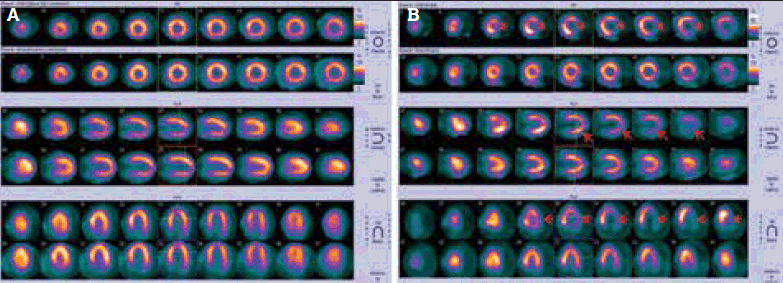
Figure 1. (a) Standard MPS display format. (b) Lateral wall has reduced radiotracer activity in the stress phase (arrows) and normal perfusion in the rest phase, indicating ischaemia
When would an MPS be more suitable than an exercise stress test?
Myocardial perfusion scans have a higher sensitivity and specificity (89%, 75%) for angiographically significant CAD than EST (67%, 70%).3 The diagnostic increment of MPS over EST is maximal in patients with an intermediate pre-test probability of CAD. Consensus appropriate use criteria4 and methodology5 for MPS have been developed by the American College of Cardiology and other professional societies, and their recommendations for more common clinical scenarios are summarised in Table 1.
Table 1. A summary of common clinical scenarios and recommendations4
| Clinical scenario | Recommendation |
|---|
| Evaluation of suspected or documented coronary disease: |
|---|
‘Ischaemic equivalent’ symptoms (eg. chest pain, chest tightness, burning, shoulder pain, palpitations, jaw pain and/or new ECG abnormalities suggestive of ischaemic heart disease and/or dyspnoea and/or worsening effort tolerance)
AND
Low pre-test likelihood of significant CAD
AND
ECG interpretable
AND
Able to exercise |
EST
The incremental diagnostic value of MPS is small and does not justify the costs and radiation dose of the procedure |
Ischaemic equivalent symptom
AND
ECG not interpretable for ischaemia (eg. left bundle branch block, digoxin therapy, Wolff–Parkinson–White syndrome) |
MPS |
Ischaemic equivalent symptoms
AND
Unable to exercise |
MPS |
Ischaemic equivalent symptoms
AND
Intermediate pre-test likelihood of significant CAD |
MPS |
Ischaemic equivalent symptoms
AND
High pre-test likelihood of significant CAD |
Coronary angiography |
| EST non-diagnostic or equivocal or suspected to be false-positive |
MPS |
| Acute chest pain |
Do not initiate any investigations; send to hospital urgently |
| Asymptomatic, with low pre-test probability of significant CAD |
No further investigations necessary |
| Asymptomatic, with intermediate or high pre-test probability of significant CAD |
MPS |
| New onset or newly diagnosed heart failure |
MPS |
| Coronary stenosis of uncertain significance |
MPS |
| High coronary calcium score (Agatston score >400) or high risk with Agatston score between 100 and 400 |
MPS |
| Normal EST or MPS less than 2 years ago with intermediate-to-high risk, but asymptomatic |
No further investigation necessary |
| Within 3 months of acute coronary syndrome (ACS) with no recurrent symptoms |
No further investigation necessary |
| Within 3 months of ACS and unstable symptoms |
Coronary angiography |
| Post-revascularisation with new ischaemic equivalent symptoms |
MPS |
| More than 2 years after angioplasty/stenting or more than 5 years after coronary artery bypass surgery |
MPS |
|
Preoperative evaluation for non-cardiac surgery:
|
|---|
| Intermediate or high risk surgery or vascular surgery planned with one or more clinical risk factors |
MPS |
| Low-risk surgery or no peri-operative risk factors (eg. ischaemic heart disease, prior heart failure, cerebrovascular disease, diabetes requiring insulin or creatinine over 0.12 mmol/L) |
No further investigations necessary |
Why do MPS and coronary angiography sometimes produce seemingly different results?
Myocardial perfusion scans provide a physiological, semi-quantitative assessment of perfusion (ie. tissue blood flow) whereas angiography provides an anatomical, and usually non-quantitative, assessment of the coronary artery lumen. The apparent severity of angiographic stenosis may not correlate with the adequacy of tissue blood flow under stress conditions (perfusion reserve) since it may not take sufficient account of sequential lesions or the presence or adequacy of collateral supply.6 In patients with anginal symptoms and angiographically documented multi-vessel CAD, MPS can help identify the culprit lesion to target for revascularisation.
Conversely, numerous studies and meta-analyses have shown that a normal MPS portends a very low likelihood of acute coronary events for the subsequent 5 years; this benign prognosis is independent of coronary angiographic findings.7
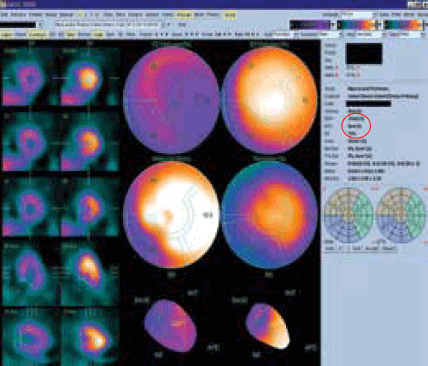
Figure 2. Left ventricular wall motion and systolic function can be analysed with an ECG-gated study. EDV, ESV and EF are calculated and displayed (circled)
Is the CT done for attenuation correction the same as diagnostic chest CT?
No. The CT scan obtained for AC covers a limited field of view (which does not encompass the whole thorax, Figure 3a), does not employ intravenous contrast, and is obtained with the patient breathing freely (rather than during a breath-hold). Hence, it is not comparable to (and is not a substitute for) diagnostic CT. However, it is sufficient to permit a visual assessment of the extent and severity of coronary artery calcification (Figure 3b), which is an independent risk factor for adverse coronary events.8 In addition, it may incidentally identify hitherto unsuspected extracardiac abnormalities such as pulmonary nodules (Figure 3c), lymphadenopathy and breast lesions.
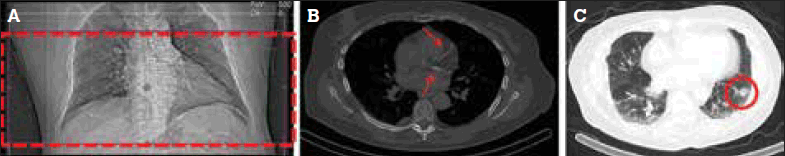
Figure 3. CT for AC has a limited field of view (box) (a) but may identify coronary calcification (arrows) (b) and pulmonary nodules (circle) (c)
How does MPS compare to stress echocardiography?
Myocardial perfusion scans and stress echocardiography (utilising exercise or dobutamine as the stressor) are the most widely used non-invasive imaging methods for assessing myocardial ischaemia. While they have similar accuracy,9 each has relative advantages and disadvantages. Although stress echocardiography does not use ionising radiation, it is highly operator-dependent and is more technically difficult in obese patients, patients with chronic obstructive lung disease and patients with resting wall motion abnormalities (eg. left bundle branch block, previous myocardial infarction).
If patients are unable to exercise, dobutamine stress echocardiography (DSE) can usually be undertaken. However, dobutamine is associated with a higher incidence of palpitations (29%), chest pain (31%), ischaemic ECG changes and significant arrhythmias than adenosine.5
Are the precautions and contraindications the same as for angiography or CT?
No. Patients who receive iodinated IV contrast for a CT scan or coronary angiogram often report a flushing sensation accompanying the injection of contrast. Intravenous contrast also carries a low risk of nephrotoxicity and allergic reactions. As the radiotracer does not contain iodine, this reaction does not occur with MPS. Likewise, renal impairment and contrast allergy are not contraindications to MPS.5 Allergic reactions to MPS agents, although reported, are exceedingly rare.
What does the patient need to know before the test?
Before MPS, the patient must fast for 2 hours. Caffeine antagonises the effect of adenosine and caffeine containing foods and beverages (chocolate, coffee, tea, cola and energy drinks) should be omitted for 12 hours before the test for patients who are to receive adenosine.5 Oral dipyridamole (eg. Asasantin®) potentiates the atrioventicular blocking effect of adenosine and should be discontinued for 72 hours before an adenosine infusion; aspirin can be substituted. Cardiac-related medications (eg. angiotensin-converting enzyme inhibitors, beta-blockers, nitrates, statins, calcium-channel blockers, diuretics, aspirin, clopidogrel and warfarin) need not be stopped to avoid precipitating acute cardiac events.3
Having the MPS on the usual medical therapy then provides a useful ‘real-life’ assessment of myocardial ischaemia.10
During the procedure, which takes about 2 hours in total, the patient will have an IV line inserted (for the injection of the radiopharmaceutical and, if pharmacologic stress is to be used, the stress agent). They will have continuous ECG monitoring throughout the stress component.
Patients may experience transient side effects from the pharmacological stressor. Adenosine has a much shorter half-life than dipyridamole (several seconds compared to 20 minutes) and hence is often preferred for this reason. The most common side effects from adenosine (and frequency of these occurrences) are listed in Table 2.
Table 2. The most common side effects of adenosine infusion5
| Side-effect | Frequency |
|---|
| Minor flushing |
35% |
| Chest tightness* |
25% |
| Mild shortness of breath |
20% |
| Dizziness |
7% |
| Nausea |
5% |
| * Chest discomfort is non-specific and is not necessarily indicative of angina. Adenosine may stimulate nociceptors within the chest to produce this effect, which is entirely separate from myocardial ischaemia5 |
Both adenosine and dipyridamole may exacerbate asthma (by producing bronchospasm). Thus, neither should be given to patients with a relevant history. If a pharmacological stress agent is required, then dobutamine is preferred.
Case report
A diabetic, hypertensive, emphysematous man, 61 years of age, saw his general practitioner because of increasingly frequent exertional chest pain. He had stopped smoking several years earlier and his father had a myocardial infarction at 54 years of age. A resting ECG was normal. He was too short of breath for an exercise test so underwent an adenosine MPS. This showed a significant perfusion defect of the distal anterior wall, apex and septum which was predominantly reversible (Figure 4a), confirmed with automated analysis (Figure 4b). Coronary angiography showed 95% stenosis in the mid left anterior descending coronary artery (Figure 4c), which was stented (Figure 4d).
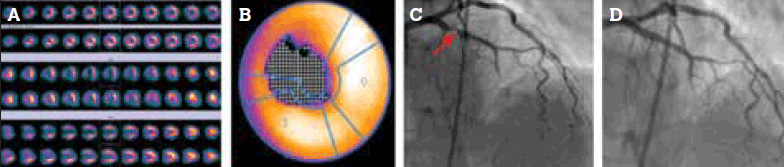
Figure 4. Ischaemia of apical, distal anterior and septal segments on (a) MPS; (b) automated analysis; and (c) coronary angiography (with arterial stenosis indicated by arrow). The lesion was stented (d)
Seven months later, he had recurrent chest pain. Another adenosine MPS showed normal perfusion, consistent with successful revascularisation (Figure 5).
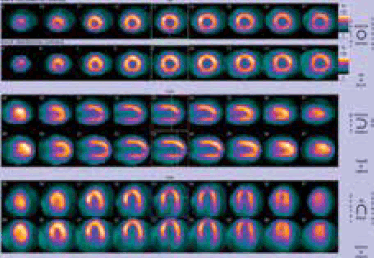
Figure 5. Normal stress perfusion seven months after the intervention
With this information, his doctor advised continuation of medical therapy and initiated investigations for non-cardiac causes of chest pain.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.