Challenge 1 – Non-adherence
Typically, multidrug regimens are necessary among patients with T2DM to manage hyperglycaemia and the associated risk factors of hypertension and dyslipidaemia.1 In these patients, non-adherence is a major concern. It has been demonstrated that non-adherence is higher among patients with diabetes than those with other common conditions (Figure 1).2 Prospective studies show that 15–39% of patients are non-adherent to oral antidiabetic drugs.3 Although patients self report a 94% adherence rate to sulphonylureas, the actual adherence rate found when electronic monitoring is used is around 75%.3
Unreported side effects and a lack of belief in immediate or future benefits are significant predictors of suboptimal adherence.1 Pharmacological therapy can be wearisome, expensive and without any symptomatic benefit. Another factor that may influence adherence is the complexity of the dosing regimen. A prospective assessment of self reported medication adherence conducted in 11 896 individuals with T2DM revealed reduced compliance with twice daily (p<0.05) and three times daily dosing (p<0.01) compared with once daily dosing.4 Socioeconomic issues, ethnicity, patient education and beliefs, and poor social support are also predictors of non-adherence.5 Declining cognitive function, polypharmacy, and poor vision among elderly patients increase the risk of medication errors which can also result in non-adherence.6–8
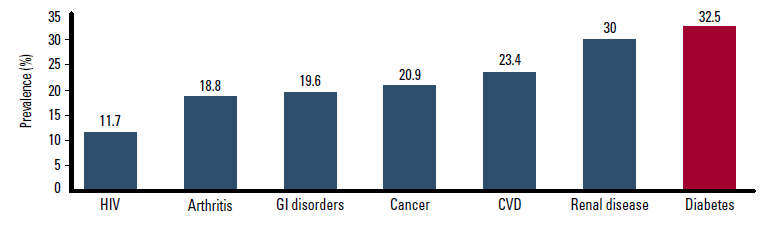
Figure 1. Non-adherence in common conditions2
Implications for management
Non-adherence with medication is associated with a statistically significant increase in hospitalisation and mortality rates, in addition to an increase in hypertension, LDL-cholesterol and HbA1c (Table 1).9 Each 10% increase in oral antidiabetic drug adherence is associated with a 0.1% decrease in HbA1c.10
Strategies are needed to increase medication adherence so patients with diabetes can realise the full benefit of prescribed therapies and potentially reduce adverse outcomes. Adherence is impaired by fragmented medical service and is likely to be improved if the patient is seen by the same trusted practitioner regularly.11 Clinicians need to implement strategies that meet the needs and concerns of individual patients. Simple measures that clinicians can undertake include:
- building rapport with patients
- working with diabetes educators, dieticians and practice nurses to reinforce messages about the importance of adherence
- monitoring repeat prescriptions
- recommending dosing aids
- explaining the progressive nature of T2DM, and that therapy will probably need to be escalated over time
- simplifying treatment regimens (eg. using fixed dose combinations where appropriate and available)
- arranging a Home Medicines Review.
Table 1. Association between medication adherence* and outcomes9
| Outcome | Adherent patients (n=9076) | Non-adherent patients (n=2456) | p value |
|---|
| All cause mortality, % |
4.0 |
5.9 |
<0.001 |
| All cause hospitalisation, % |
19.2 |
23.2 |
<0.001 |
| Mean BP, mmHg
|
131.4
74.2
|
132.1
75.8
|
0.09
<0.001
|
| Mean LDL-C, mmol/L |
2.21 |
2.34 |
<0.001 |
| Mean HbA1c |
7.7 |
8.1 |
<0.001 |
| * Measured as the proportion of days covered for filled prescriptions of oral hypoglycaemic agents, antihypertensive agents and statins |
Home Medicines Review
Subsidised by the Australian Government, Home Medicines Review (HMR) is a service geared toward optimising medication use and health outcomes for community based patients. The service is initiated by GPs and conducted by pharmacists accredited to undertake medication reviews. General practitioners and pharmacists are reimbursed for providing HMR and there is no charge to the patient.12 The major benefits of the service are acquisition of medicine information, reassurance, feeling valued and cared for, and willingness to advocate medication changes to the GP.13 Despite having been shown to successfully identify medication related problems and improve drug knowledge and adherence, uptake of this service has remained low.14
Challenge 2 – Cardiovascular disease and glycaemia
Cardiovascular disease (CVD) is the major cause of death in people with diabetes, accounting for approximately 50% of all fatalities.15 The Australian Diabetes, Obesity and Lifestyle (AusDiab) study found in 5 years of follow up, that 65% of all CVD deaths occurred in people with known diabetes, newly diagnosed diabetes, impaired fasting glucose, or impaired glucose tolerance at baseline. Among people with known diabetes, the risk of death from CVD was significantly greater than that found in those without diabetes.16,17 A United Kingdom study noted that after an average of 6 years follow up, patients with known or undiagnosed diabetes had a greater risk for coronary heart disease (CHD), CVD events, and all cause mortality than patients without diabetes.18 Among men, a gradient of increasing rates through the distribution of HbA1c concentrations from HbA1c levels of 5% was apparent for all endpoints. Among women, the odds ratio for CVD or CHD did not increase significantly until HbA1c reached 6%.18
Implications for management
In 2009, the Australian Diabetes Society (ADS) recommended more aggressive glycaemic targets in newly diagnosed patients with diabetes. It is recommended to treat those without a history of CVD who are managed with lifestyle advice and metformin alone to a target HbA1c of ≤6.0%. In those on additional medication, excluding insulin, the target is ≤6.5%.19 However, recent large scale trials sound a note of caution regarding the benefits of intensive glycaemic control on macrovascular outcomes in patients with established CVD.20 The Action in Diabetes and Vascular Disease (ADVANCE) trial found that intensive glycaemic control (HbA1c 6.5% in the intensively controlled group vs 7.3% in the control group) made no significant difference in macrovascular outcomes, but did reduce the incidence of microvascular events.21 The Action to Control Cardiovascular Risk in Diabetes Study (ACCORD), which aimed for an HbA1c of 6.0% in the intensively controlled arm, was stopped early due to a higher number of cardiovascular events. The intensively controlled arm achieved an average HbA1c of 6.4% and demonstrated an increased mortality rate compared with those in the control group (HbA1c 7.5%).22 The exact aetiology of this increase in mortality has not been established.20 Both the ADVANCE and ACCORD trials enrolled high risk patients who had been treated for 8 and 10 years respectively, and around a third had a history of macrovascular disease.23 These studies have prompted further research to explore the safety of the many different hypoglycaemic agents in patients with a history of CVD.24 The ADS recommended HbA1c target for patients with longer standing diabetes (more than 10 years) or with known CVD is ≤7%, reflecting concerns raised by ADVANCE and ACCORD.19 It is important to realise that newer incretin based therapies such as DPP-4 inhibitors and GLP-1 agonists were not included in either of these two large trials and their impact on cardiovascular risk is yet to be fully defined.
The foundations of managing diabetes and CVD are diet, physical activity and weight control. However, eventually most people will also require drug treatment. The STENO-2 study addressed multiple risk factors among patients with T2DM, by controlling HbA1c, blood pressure and lipids, and a regimen of aspirin and an angiotensin converting enzyme inhibitor, a healthy diet, physical activity and smoking cessation. Long term management more than halved the risk of CVD.19 There is also evidence that statin therapy markedly reduces macrovascular events in T2DM.20,21
When prescribing diabetes specific drugs for patients with T2DM and CVD, clinicians need to be familiar with the cardiovascular efficacy and safety of these agents. Table 2 shows recommendations according to cardiovascular status.22 Data suggest that the combination of glibenclamide and metformin should be avoided in the long term management of T2DM patients with proven CVD and that glitazones should be avoided in those with heart failure. Acarbose may have a role in managing patients with CVD.
In the recent Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial of more than 12 000 people who had cardiovascular risk factors and either pre-diabetes or T2DM, basal doses of insulin glargine given over more than 6 years had a neutral effect on CVD outcomes compared with other treatments.
Incretin therapies, although not approved as monotherapy, could be an alternative adjunct therapy in patients with cardiovascular risk but data from prospective trials are still awaited.18 It is clear that customised regimens are necessary for the optimal management of T2DM patients with heart disease.
Table 2. Diabetes-specific drugs recommended according to
cardiovascular status
| No known vascular disease | Stable coronary heart disease | Acute coronary syndrome and myocardial infarction | Chronic heart failure |
|---|
| Intensive treatment with metformin, sulphonylurea, or glitazone |
Intensive treatment, consider glitazone |
Intensive treatment with multi-dose insulin, glitazone |
Metformin, insulin |
| Adapted from White A, McKay, GA, Fisher M. Drugs for diabetes. Part 9. Prescribing for patients with cardiac disease. Br J Cardiol 2012;19:85–9 |
Challenge 3 – Impact of renal impairment
According to the NEFRON study, almost one in every two patients with T2DM in Australia has chronic kidney disease (CKD).30 The United Kingdom Prospective Diabetes Study (UKPDS) suggested that nearly 25% of patients develop microalbuminuria within 10 years of diagnosis of T2DM (Figure 2).31
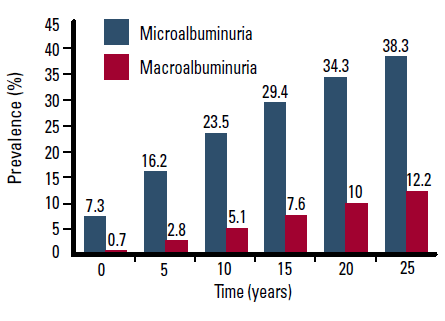
Figure 2. Prevalence of microalbuminuria and macroalbuminuria with increasing duration of T2DM31
Implications for management
Subanalyses of the ADVANCE trial demonstrated that the presence of baseline albuminuria is a risk marker for complications in diabetes (Figure 3).32 Among patients with CKD, the greatest risk of progression and CVD is a combination of low estimated glomerular filtration rate (eGFR) and albuminuria over either risk factor alone.33
Preventing or slowing the progression of renal disease is an important facet of managing patients with T2DM. Steno-2 demonstrated multifactorial risk management, as described above, halved nephropathy among patients with T2DM and microalbuminuria.25 The ADVANCE trial demonstrated, compared with standard glycaemic control, tight glycaemic control results in a significant reduction in renal events, including new or worsening nephropathy, new onset microalbuminuria and the development of macroalbuminuria.21 Further analyses of ADVANCE found that the separate effects of blood pressure lowering with perindopril/indapamide and intensive glucose control appeared to be additive. Compared with neither intervention, combination treatment reduced the risk of new or worsening nephropathy by 33% (p<0.005); new onset microalbuminuria by 54% (p<0.0001); and new onset microalbuminuria by 26%. In addition, combination treatment was associated with an 18% reduction in the risk of all cause death (p=0.04).34
Renal function is an important factor to be considered in the decision to prescribe diabetes specific therapy. The risk of lactic acidosis with metformin is controversial, however, recommendations suggest the dose of metformin should be halved in patients with a creatinine clearance of 30–60 mL/min and discontinued in those with levels <30 mL/min.35 However, metformin remains a potent diabetes drug, and there is no clear evidence that prescribing it in this patient population is harmful.36
In comparison with short acting sulphonylureas, long acting sulphonylureas are more likely to cause hypoglycaemia and the risk is increased in patients with renal impairment and/or advanced age. Shorter acting agents, initiated at a low dose and increased gradually, are a more appropriate choice in patients with renal impairment,35 and probably also more appropriate for the elderly.
Dose reductions are recommended for sitagliptin in patients with moderate-to-severe CKD and in patients with end stage renal disease on haemodialysis.37 As they are predominantly cleared by renal excretion, vildagliptin and saxagliptin are not recommended in patients with moderate or severe renal impairment or in patients on haemodialysis. Dose reductions are not necessary in patients with mild renal impairment.37 Linagliptin has only 5% renal excretion and is cleared predominantly through the enterobiliary system, making it safe to use in renal and hepatic failure.38 The ability of incretins such as DPP-4 inhibitors and GLP-1 analogues to protect against the development or progression of diabetic kidney disease is yet to be defined in prospective clinical trials.
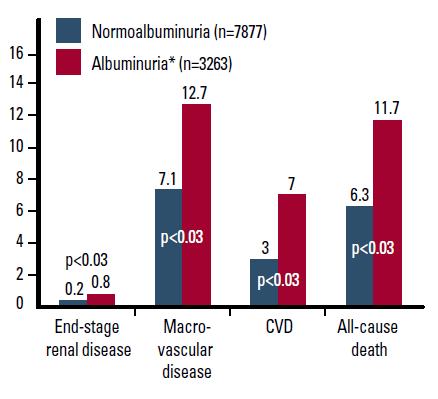
Figure 3. Adjusted# risks of end stage kidney disease, macrovascular events, cardiovascular death, and all cause death by baseline albuminuria level32
# Adjusted for age, gender, HbA1c, systolic BP, diastolic BP, LDL-C, HDL-C, triglycerides, BMI, eGFR, current smoking and current alcohol use
* 88% had microalbuminuria and 12% had macroalbuminuria at baseline
Key points
- Non-adherence to medications is relatively common among patients with T2DM.
- The risk of non-adherence increases with the number of medications prescribed and higher dosing frequency.
- Clinicians need to be familiar with the cardiovascular safety of oral antidiabetic drugs.
- Almost one in every two patients with T2DM in Australia has chronic kidney disease.
- Tight glycaemic control combined with lowering blood pressure results in a significant reduction in renal events.
- Renal function is an important factor to be considered in decisions about choice of oral antidiabetic drugs.
- The management of complex T2DM patients is ideally performed by GPs, balancing the risks and benefits of each treatment decision in light of the patient’s glycaemic, cardiovascular, renal status and motivation.
Competing interests: Mark Kennedy has received payment for consultancy, lectures and educational presentations from AstraZeneca, Bristol-Myers Squibb and Sanofi Diabetes. Adam Roberts has received payment for lectures, travel and accommodation from Eli Lilly, Merck Sharp & Dohme, Boehringer and Novo Nordisk.
Provenance and peer review: Not commissioned; externally peer reviewed.