Fragile X syndrome and associated conditions
PRACTICE POINT
General practitioners (GPs) can order a test for fragile X syndrome (FXS) for the following people:
- Individuals with intellectual disability (ID), developmental delay (DD) or autism spectrum disorder (ASD).
- Individuals seen for reproductive counselling who have a family history of FXS (or related conditions, such as fragile X-associated primary ovarian insufficiency [FXPOI]) or undiagnosed ID.
- Women with evidence of primary ovarian insufficiency (associated with elevated follicle-stimulating hormone [FSH]).
- Adults with tremor or cerebellar ataxia of unknown origin to diagnose fragile X-associated tremor/ataxia syndrome (FAXTAS). Mean onset of FAXTAS is ~60 years of age but can present before age 50.
What do I need to know?
FXS
FXS is the most common inherited cause of ID, and the second most common cause of ID overall (after Down syndrome). FXS affects approximately one in 3600 males and one in 6000 females.
FXS presents clinically with a wide range of symptoms, including global DD; difficulties with learning, speech and language; problems with coordination and sensory overload; and notably a range of emotional and behavioural difficulties.
FXS follows an X-linked dominant inheritance pattern and is caused by an increase in length of a CGG trinucleotide repeat in the FMR1 gene on the X chromosome. The length of the FMR1 CGG repeat is divided into four categories (Figure 1). The longer the repeat, the more likely the individual will have symptoms of FXS.
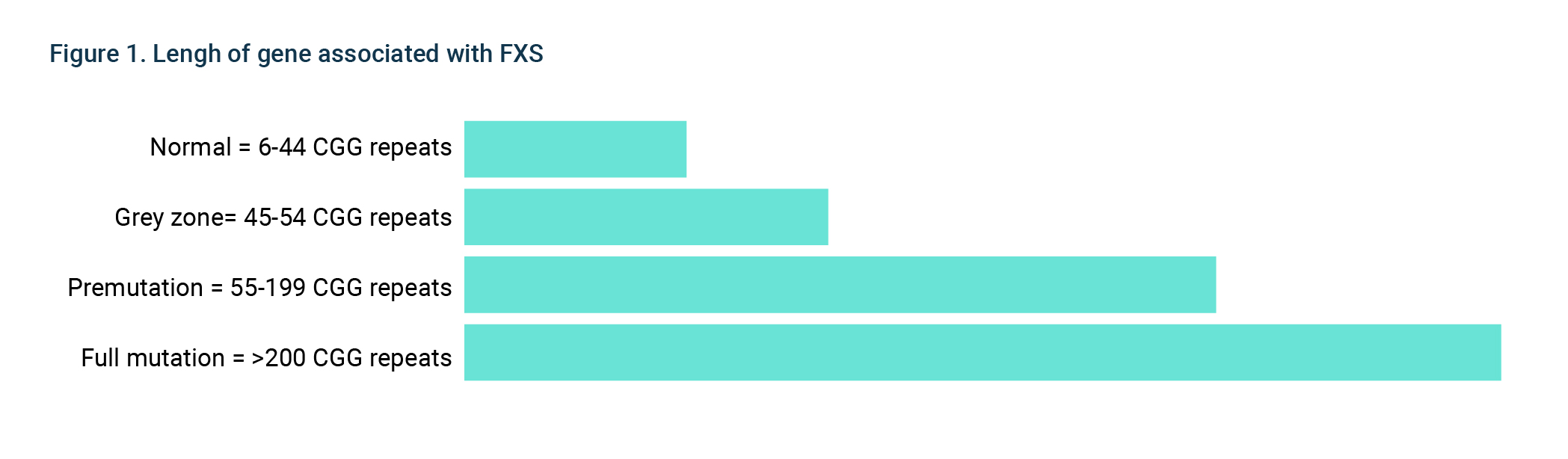
Females who are premutation carriers (55 – 199 CGG repeats) can have a child affected with FXS. This is because the repeat length can get longer when passed from mother to child: this lengthening only occurs in women. Therefore, it is very unlikely males who are premutation carrier of FXS would have a child with FXS.
Associated conditions
Females who are premutation are at increased risk of FXPOI. Both males and females with a premutation are at increased risk of fragile X-associated tremor/ataxia syndrome (FXTAS) later in life with the risk being higher for males than females.
Genetic testing
Chromosome microarray (CMA) is now considered a first-line genetic test for the investigation of DD or congenital abnormalities. However, CMA does not detect causing FXS, so an additional DNA test for FXS must be also ordered.
DNA testing for FXS is available with an MBS rebate when the patient:
- exhibits ID, ataxia, neurodegeneration, or primary ovarian insufficiency consistent with an FMR1 mutation
- has a relative with an FMR1 mutation
GPs should offer information on carrier screening for FXS to all couples planning a pregnancy (or who are already pregnant), regardless of family history or ethnicity. Refer to Reproductive carrier screening for more information.
When should I refer?
For symptomatic patients:
- A child with a test positive result should be referred to a paediatrician and clinical genetics team for further assessment
- An adult with ataxic symptoms and a test positive result (ie premutation carrier) should be referred to a neurologist and clinical genetics team.
- A woman with FXPOI should be referred to an obstetrician and gynaecologist and clinical genetics team.
Asymptomatic patients with a test positive result (eg received through pre-conception or prenatal or cascade testing) should be referred to genetics services.
Further reading
- Birch RC, Cohen J, Trollor Fragile X-associated disorders: Don’t miss them. Aust Fam Physician 2017;46(7):487–91. [Accessed 6 September 2022].
- Finucane B, Abrams L, Cronister A, et Genetic counseling and testing for FMR1 gene mutations: Practice guidelines of the National Society of Genetic Counselors. J Genet Couns 2012;21(6):752–60.
- RANZCOG Genetic carrier screening. 2019.
- Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genetics in Medicine (2021) 23:1793–1806
- Miller DT, Adam MP, Aradhya S, et Consensus statement: Chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet 2010;86(5):749–64.
- Department of Medicare Benefits Schedule (MBS) Online. Canberra: DoH, 2022. [Accessed 11 February 2022].
Resources for patients
Information
Support