Case study illustrating principles of management
SV, a single woman, 20 years of age, presented to her OST prescriber, requesting to cease Suboxone (buprenorphine–naloxone) after discovering she was 4 weeks pregnant. She was concerned that continuing Suboxone would affect her pregnancy and unborn child. She had a past history of intravenous heroin use, commencing at 18 years of age, and had been stable on Suboxone for the past 12 months. She had no other medical problems and reported no current substance use.
- Engaging a woman in treatment through a nonjudgemental approach, empathic listening and careful exploration of her specific concerns is a starting point to forming a therapeutic alliance
- Accurate information provision is essential in supporting a patient in making an informed decision
- Respecting the patient’s choice and supporting her decision-making provides an opportunity for her to remain engaged in treatment for ongoing monitoring and follow-up
- Referring her to a specialist drug and alcohol service for a second opinion and secondary consultation may be beneficial
- Facilitating the patient’s linkage with the appropriate antenatal service may provide additional support
- Regular communication between the general practitioner, antenatal service and drug and alcohol service is crucial to providing holistic and integrated care
SV’s concerns included the stigma associated with obtaining OST from her community pharmacy as a pregnant woman, concerns about the teratogenic effects of Suboxone and the effects of Suboxone withdrawal on her baby following delivery. Her GP acknowledged and allayed her anxieties by providing information regarding the rationale for OST as the recommended treatment for opioid dependence during pregnancy.
She explained the likelihood of neonatal abstinence syndrome and treatment of it. She also explained that a change to the mono form of buprenorphine (Subutex) was standard practice in women on Suboxone, given the evidence for safety in pregnancy of the mono preparation. Finally, the prescribing GP explained that there was no evidence of teratogenicity associated with methadone or buprenorphine.
Her GP advised SV that if she was insistent on withdrawing from OST, the risk associated with medically supervised withdrawal would likely to be lower during the second trimester. SV agreed to be referred to a specialist alcohol and drug service, where the general practitioner’s recommendations were reinforced. The service offered to provide secondary consultation around the dosages during the antenatal period. SV agreed to continue on opioid treatment.
SV agreed to be referred to a specialist antenatal service, where her broader antenatal needs were addressed and she was provided with more information about the management of neonatal abstinence syndrome.
Opioid use in pregnancy – background
Heroin use, particularly dependent heroin use, is associated with a broad range of obstetric complications, including intrauterine growth retardation, pre-term labour, placental abruption, intrauterine passage of meconium, neonatal abstinence syndrome (NAS), and foetal and neonatal death. These risks may be related to repeated exposure of the foetus to opiate withdrawal, as well as the effects of withdrawal on placental function.2 Ongoing heroin use is often associated with inadequate antenatal care and other physical health complications such as poor nutrition, blood-borne virus exposure and overdose. In addition, the psychological and social sequelae that often accompany ongoing illicit substance use, including abuse, financial hardship and unstable accommodation, can further complicate the pregnancy.3
Despite such risks, pregnant women are often highly motivated to modify their behaviour,1,4 making pregnancy an ideal time to engage a drug-dependent woman in specific treatments that will benefit her and her unborn child.
Assessment of the opioid-dependent pregnant woman
As with other complex presentations, opioid-dependent pregnant women require a holistic assessment and the development of a comprehensive management plan that addresses psychosocial, mental and physical health care needs. In addition to consideration of opioid-substitution therapy (OST), referral to an antenatal service with experience in the specialist management of these patients is usually required as well as engagement of psychosocial support agencies (see Figure 1).
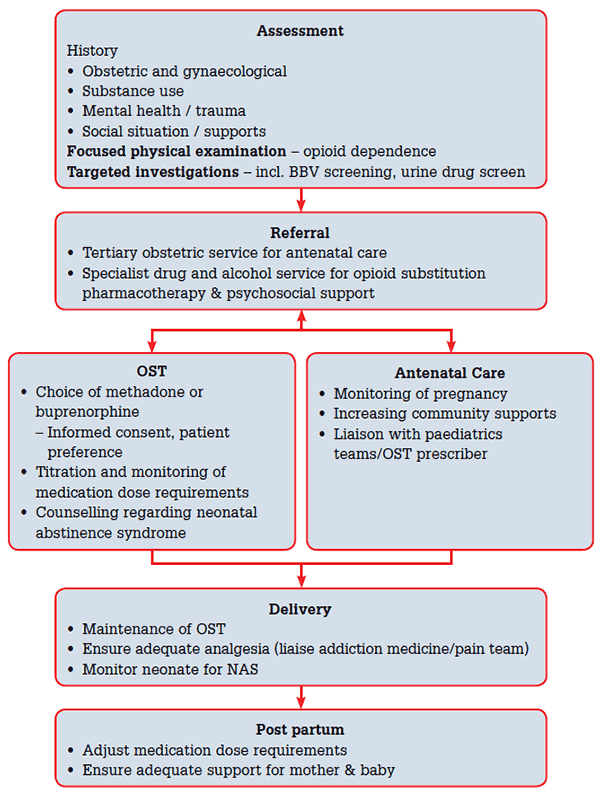
Figure 1. Clinical approach to opioid dependent women
A baseline assessment should focus on establishing rapport and building a therapeutic alliance with the patient, whilst gathering information to optimise treatment. A thorough history of current opioid and other substance use, including use of nicotine, alcohol and other drugs (prescribed, illicit and over-the-counter) can help guide treatment. Assessment also needs to elicit obstetric and gynaecological history including previous pregnancies, antenatal complications and terminations. An overview of personal and social circumstances will help clarify supports and vulnerabilities. An open and empathic attitude is helpful when discussing sensitive issues, such as feelings and fears about the pregnancy, the baby and the future (see case study). Targeted physical examination should focus on features of opioid use. Investigations such as blood-borne virus serology (hepatitis B, hepatitis C, human immunodeficiency virus) and a urine drug screen can guide further management. Many of these women may present late to antenatal services and thus miss out on routine monitoring, so it may be useful to consider offering an 11–13-week ultrasound scan as well as the recommended antenatal blood tests.
Pregnancy and opioid-substitution therapy
Goals of OST include limiting foetal exposure to peaks and troughs of short-acting opioids such as heroin and stabilisation of the intrauterine environment. Opioid-dependent women who receive substitution therapy during their pregnancy are more stable psychologically and physically, receive more comprehensive antenatal care, and have better neonatal outcomes than women who are not on OST.5 These benefits are worth reiterating to the patient, while exploring attitudes towards maintenance therapy, and addressing any misconceptions that might be a barrier to medication-assisted treatment.
Methadone or buprenorphine
Both forms of OST that are prescribed in pregnancy – the mu opioid receptor full agonist methadone and partial agonist buprenorphine – cross the placenta and may have an effect on the neonate.
With a background of 5 decades of experience and a substantial literature base supporting its benefit in pregnant women, methadone maintenance therapy (MMT) – usually involving a supervised dosing program using methadone solution – is the most widely used approach to managing opioid dependence in pregnancy.6 Methadone treatment is associated with improved obstetric care, lower rates of HIV infection, decreased risk of pre-eclampsia, and reduction in foetal exposure to cycles of heroin intoxication and withdrawal.6 It has been the gold standard treatment for opioid-dependent pregnant women.
Sublingual high-dose buprenorphine is a relatively new drug compared to methadone, and is available in Australia in ‘mono’ tablet form and as a film preparation in a 1:4 combination with naloxone (to deter injection misuse). Despite a smaller literature base, emerging evidence supports the use of buprenorphine maintenance therapy (BMT) in pregnancy. Unlike methadone, current Australian product information for sublingual high-dose buprenorphine carries a contraindication for pregnancy and breastfeeding, although it is in the same pregnancy safety category as methadone (Category C). To date, the largest randomised trial looking at maternal OST is the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study. This US-based project demonstrated that buprenorphine exposed neonates required, on average, 89% less morphine for NAS and substantially shorter hospital stays.7 Furthermore, BMT has been introduced in Europe and the USA as ‘office-based’ treatment, prescribed in primary care rather than specialist drug treatment clinic settings, mainly due to its ease of dosing, lower risk of overdose and fewer drug interactions compared to methadone.7 These benefits need to be weighed up against treatment retention, which the MOTHER trial found was higher in patients on MMT. As with methadone, long-term data regarding the neurodevelopmental effects of exposure to buprenorphine in utero are lacking.
As there is insufficient data surrounding the safety of naloxone in pregnancy, only the mono (buprenorphine only) form is currently offered to pregnant patients for whom BMT is indicated. Women who become pregnant while receiving buprenorphine-naloxone should be converted to the buprenorphine-only preparation. A change to mono-buprenorphine may necessitate an increase in levels of dosing supervision, given the higher risks of injection abuse of this preparation compared to the buprenorphine-naloxone combination.
In summary, both methadone and buprenorphine treatment are associated with positive maternal and foetal outcomes in opioid-dependent women, by reducing the risk of complications associated with illicit opioid use. While methadone has been the first-line option in most women and may have better treatment retention, emerging evidence suggests that buprenorphine is as effective as methadone in preventing relapse to heroin use, and may be associated with fewer neonatal complications.
Care during pregnancy
Patients should be reviewed regularly during the course of their pregnancy for several reasons. Careful titration of OST is required to manage opioid craving and physiological withdrawal. Opioid withdrawal as a result of insufficient dose of maintenance medication is associated with foetal stress, and relapse to high-risk substance use. Dose increments are usually required as the pregnancy progresses, due to increases in drug metabolism and blood volume, occurring particularly in the third trimester.8
Frequent reviews through pregnancy will also assist in monitoring for problems associated with dosing, such as vomiting. Many women have difficulties with vomiting through pregnancy, and this can impact on the amount of medication absorbed, dose required or withdrawal symptoms. If there is repeated vomiting, consideration needs to be given to anti-emetic medication, and management of hyperemesis.
Finally, close monitoring of mental state and psychosocial stressors are indicated in pregnancy. In preparation for motherhood, treating GPs and other members of the treating team may need to provide increased psychosocial support. Worsening psychological symptoms that may trigger relapse into high-risk drug use will benefit from early intervention. The treatment team of prescriber and dispenser of opioid pharmacotherapy are well placed to respond to psychological destabilisation in pregnancy.
Neonatal abstinence syndrome
While maternal OST with methadone or buprenorphine improves foetal and pregnancy outcomes, any long-term opioid therapy carries a risk of opioid withdrawal for the newborn, or NAS. The risk of NAS must be clearly explained to women as part of the informed consent process prior to commencement of OST.
Features of NAS usually arise within the first 48–72 hours of birth and, depending on whether the mother was maintained on buprenorphine or methadone, the syndrome may last from a week to several weeks.8 NAS is characterised by central and autonomic hyperactivity, and is associated with difficulty feeding, poor suckling, irritability, hyperactivity, sleep disturbances and a high-pitched cry.8 Neonatal seizures are uncommon in NAS, and occur in about 5% of cases.9 Neonates at risk of NAS are usually monitored closely in special care nurseries for a prolonged post-partum period, with most infants exhibiting mild-to-moderate symptoms managed with general supportive measures, ideally within a unit with experience in the care of neonates born to opioid dependent mothers. Pharmacological intervention with oral morphine is usually guided by careful observation and standardised scoring, with dose and duration of treatment based on presence of complications such as irritability, convulsions, feeding problems, weight loss and sleep disturbance.
Current evidence suggests that between 50–80% of neonates with in utero methadone exposure show signs of NAS; whereas about 47% of buprenorphine-exposed infants may display these signs.6 There is also some evidence that NAS due to buprenorphine exposure may be less severe, require less medication and a shorter hospital stay compared with NAS due to methadone exposure.6 There is currently no evidence to suggest a link between the maternal dose of opioid maintenance treatment and the severity of NAS.2,7,8 NAS is highly variable in severity, and affected by a range of factors in addition to the opioid-maintenance agent, including maternal smoking, ongoing heroin use and concurrent benzodiazepine dependence.2
Delivery and care during the post-partum period
Good communication between the obstetrics team and the opioid prescriber through the course of the pregnancy is vital, particularly during the intrapartum and post-partum period. Current practice supports continuation of OST during admission for delivery. This may pose a challenge for pain management during delivery, as patients receiving OST often require higher than usual doses of opioids to achieve analgesia. Thus, other forms of analgesia may be indicated, such as inhaled nitrous oxide and spinal or epidural anaesthesia.2
Hospital obstetric units may benefit from consultation with addiction medicine or pain teams in tertiary hospitals where such services are available. In addition, paediatric staff should be aware of all infants exposed to long-term maternal opioid treatment.
Women can be advised that methadone or buprenorphine concentrations in breast milk are low irrespective of the maternal dose2 and should be encouraged to breastfeed while continuing OST if there are no other contraindications. Dose requirements may decrease during the post-partum period.2 It is important to closely monitor infants for symptoms and signs that the dose may need downward titration, with particular attention to features of toxicity, such as oversedation. This is also an opportune time to assist the mother with accessing psychosocial supports, and to begin discussion of contraceptive options.
Key points
- Opioid-substitution therapy is one aspect of a comprehensive management plan to address an opioid-dependent pregnant woman’s healthcare needs.
- Women are often highly motivated to address health behaviours during pregnancy, so this presents an opportunity to offer treatment for substance use.
- Methadone is an established and safe opioid-substitution treatment during pregnancy. Buprenorphine can be considered as a second-line alternative, with specialist addiction medicine and obstetric advice.
- Regular review during pregnancy will ensure that patients get an adequate dose of substitution treatment.
- Women need to be informed of the risk of NAS in their infants.
- Communication between treating professionals is vital to ensure the appropriate care of mother and baby during the intra- and post-partum period.
- Managing opioid dependence in a pregnant woman can be a rewarding experience.
Competing interests: Dan Lubman has received payment for speaking honorarium from Astra Zeneca and Janssen-Cilag and has provided consultancy advice to Lundbeck. Conference travel support has also been provided by Lundbeck. Matthew Frei has received financial support for conference presentations from Reckitt Benckiser.
Provenance and peer review: Not commissioned; externally peer reviewed.