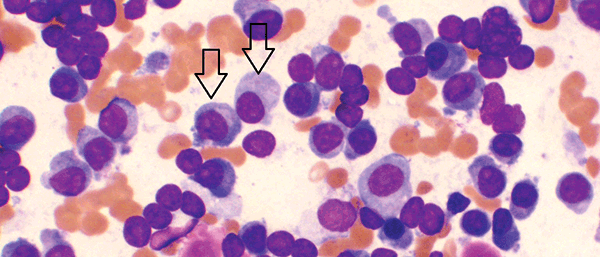
Figure 1A. Bone marrow aspirate showing increased numbers of plasma cells, including large and immature forms (arrows)
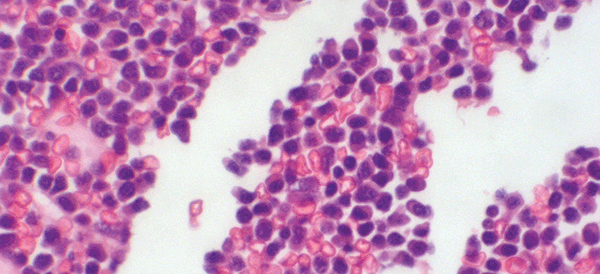
Figure 1B. Trephine showing infiltration with malignant plasma cells
MGUS and smouldering myeloma are asymptomatic with no evidence of end organ damage. Monoclonal gammopathy of undetermined significance is defined as a serum paraprotein of <30 g/L with a marrow plasma cell infiltrate of less than 10%. Smouldering multiple myeloma is typified by a serum paraprotein of >30 g/L or a bone marrow plasmacytosis of >10% (Table 1).
Table 1. When should a diagnosis of myeloma be considered?
| Finding | When to consider myeloma |
|---|
| Anaemia (normocytic or macrocytic) |
Vitamin B12, folate and iron studies normal
No history of blood loss
No haemolysis
No clear alternative explanation such as renal impairment or anaemia of chronic disease |
| Hypercalcaemia |
Parathyroid hormone appropriately suppressed
Vitamin D normal
No history of malignancy, sarcoidosis or use of medications such as thiazides |
| Renal impairment |
No clear explanation including pre-renal causes, primary renal disorders or obstructive conditions |
| Bony pain/fractures |
Evidence of bony lesions on imaging
Crush fractures in a young patient
Pathological fractures in unusual sites |
| Monoclonal paraprotein |
Usually required to confirm a diagnosis of multiple myeloma
A small proportion of cases may be non-secretory with undetectable paraprotein |
How can multiple myeloma and MGUS present?
Multiple myeloma
The clinical presentation of multiple myeloma can be extremely varied. The classical complications are often abbreviated into the acronym ‘CRAB’: consisting of hypercalcaemia, renal impairment, anaemia and bony lesions. A combination of these symptoms should heighten diagnostic suspicion for myeloma.
Anaemia is found in approximately 70% of those with newly diagnosed myeloma.1 Patients with significant anaemia may present with fatigue, dyspnoea on exertion or angina. Alternatively, an asymptomatic, but persistent, anaemia may be detected on routine bloods. The anaemia of multiple myeloma is typically normochromic and normocytic, but mild macrocytosis can also be seen. Routine haematinics are normal and rouleaux may be noted on the blood film.
Hypercalcaemia is a less frequent manifestation of myeloma at diagnosis, occurring in approximately 13% of patients.2 The serum calcium level should always be interpreted after correction for albumin. Symptomatic hypercalcaemia may present in a dramatic fashion with confusion, disorientation, muscle weakness, constipation, anorexia, polyuria and polydipsia. Profound elevations in serum calcium can rarely lead to coma or cardiac arrhythmia.3 Alternative aetiologies should be excluded with a laboratory assay for intact parathyroid hormone (PTH), which should be suppressed in response to the hypercalcaemia, and vitamin D levels. Some laboratories also offer PTH related peptide assays to exclude paraneoplastic hypercalcaemia. If PTH levels are appropriately suppressed, an alternative explanation such as myeloma must be considered.
Renal impairment occurs in 20–40% of newly diagnosed patients with myeloma, and is typically due to light chain deposition within the distal and collecting renal tubules or hypercalcaemia.4 It is most commonly detected as an asymptomatic elevation in serum creatinine, but patients may rarely present with oliguria or uraemia.
Bony pain is a relatively common symptom, particularly back pain, occurring in up to 58% of patients. Lytic lesions are detectable in almost 80% of patients at diagnosis.5 Those with more advanced disease may present with a pathological fracture from minimal trauma (Figure 2). A skeletal survey, or whole body X-ray survey, is typically employed to detect osteolytic lesions. Magnetic resonance imaging (MRI) short-tau inversion recovery (STIR) is a more sensitive investigation, and may be used to identify lytic lesions in suspicious cases when the skeletal survey fails to yield a diagnosis.6 In regions where MRI is unavailable, computed tomography (CT) may have a role in further investigating suspicious, but non-diagnostic, bony lesions seen on plain film.
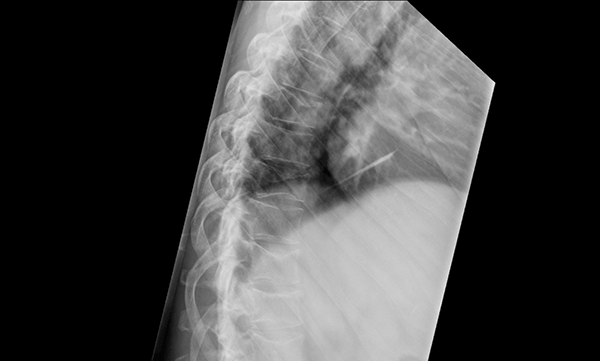
Figure 2. Thoracic spine x-ray demonstrating multiple wedge compression insufficiency fractures typical of myeloma
Less conventional presentations with myeloma include recurrent bacterial infections (defined as >2 episodes in 12 months), often due to an associated hypogammaglobulinaemia, or symptomatic hyperviscosity with confusion, visual changes, headaches and vertigo.
Smouldering myeloma and MGUS
By definition, patients with MGUS and smouldering multiple myeloma are asymptomatic. The condition may become apparent when routine blood tests reveal an elevated total protein and globulin, and subsequent serum electrophoresis demonstrates a monoclonal paraprotein. These patients may ultimately progress to symptomatic myeloma and require careful monitoring.
Smouldering multiple myeloma requires closer follow up because of a higher risk of progression (10% per year compared to 1% per year).7
What investigations are performed to make a diagnosis of multiple myeloma?
Evaluating a patient with suspected multiple myeloma involves establishing evidence of a monoclonal paraprotein with serum electrophoresis (EPG) and immunofixation, together with an FLC analysis. Serum EPG establishes the presence of a monoclonal band, while immunofixation increases diagnostic sensitivity to 93% and determines the precise nature of the paraprotein, for example, if it belongs to the IgA or IgG class and whether it involves the kappa or lambda light chains. IgM multiple myeloma is a very rare entity and the presence of an IgM paraprotein should raise suspicion of the related disease Waldenström’s macroglobulinaemia/lymphoplasmacytic lymphoma. A serum FLC analysis must always be requested, as a small percentage of patients do not have measurable disease on serum EPG and immunofixation and would be otherwise missed. The combination of these three tests has a diagnostic sensitivity of 97–98%.8 Two percent of patients have true non-secretory disease that is undetectable by any of these methods.9 The International Myeloma Working Group suggests the FLC assay can replace the traditional 24-hour urine protein electrophoresis and immunofixation as part of a diagnostic screen10; but these tests are still recommended once the diagnosis of multiple myeloma has been established.
Full blood count and film, electrolytes including urea and creatinine, calcium, magnesium and phosphate analysis should always be performed to detect evidence of end-organ disease. For prognostication purposes, serum â2-microglobulin and albumin should also be assessed, in addition to serum LDH. Imaging to detect bony lesions, typically in the form of a skeletal survey, is mandatory. A referral to a haematologist should be made in order to obtain a bone marrow aspirate and trephine, and to guide further management (Table 2).
Table 2. Relevant investigations in myeloma12
| Baseline investigations by GP | Investigations by haematologist |
|---|
Haematology
- Full blood count and blood film
Biochemistry
- Creatinine, urea and electrolytes
- Liver function tests, calcium, magnesium, phosphate, urate β2 microglobulin
- Lactate dehydrogenase (LDH)
- Serum electrophoresis and immunofixation
- Serum free light chain analysis
- 24-hour urine protein and electrophoresis studies
Imaging
- Skeletal survey (whole body X-rays)
|
- Bone marrow aspirate and trephine, including cytogenetic analysis/FISH for common translocations, immunohistochemistry and flow cytometry
- Viral serology
- Transthoracic echocardiography or gated heart pool scan (if indicated)
- MRI STIR (if indicated)
|
Diagnostic criteria
Diagnosis of multiple myeloma is based on the International Myeloma Working Group guidelines (Table 3).10 The diagnosis rests on the presence of a monoclonal paraprotein together with marrow plasmacytosis and myeloma-related end-organ damage. These are typically the ‘CRAB’ symptoms described, but recurrent bacterial infections (>2 a year), hyperviscosity symptoms and features of amyloidosis also qualify.10
Table 3. Diagnostic criteria for multiple myeloma10
| Monoclonal gammopathy of uncertain significance | Smouldering myeloma | Multiple myeloma |
|---|
| Serum monoclonal protein <30g/L |
Serum monoclonal protein ≥30g/L |
Monoclonal protein in serum and/or urine |
| Bone marrow clonal plasma cells <10% |
Bone marrow monoclonal plasma cells ≥10% |
Bone marrow clonal plasma cells or biopsy proven plasmacytoma |
| No evidence of other B cell lymphoproliferative disorders |
No myeloma-related organ or tissue impairment |
Myeloma-related organ or tissue impairment |
| No myeloma-related organ or tissue impairment |
|
|
Are there features that help determine prognosis?
Prognosis is commonly evaluated using the International Prognostic Index, which divides patients into three stages. Stage I disease, with a median survival of 62 months, is defined as those with a â2-microglobulin of <3.5mg/L and a serum albumin above 35g/L. Stage III disease, median survival 29 months, includes those with a â2-microglobulin above 5.5mg/L. Stage II disease, for patients who do not fulfil the criteria for the other stages, has a median survival of 44 months.11 There are a number of additional prognostic markers used by haematologists, including cytogenetics/fluorescence in situ hybridisation (FISH) and the plasma cell labelling index, some of which are used more commonly than others.
In MGUS, the type and quantity of paraprotein and the presence of abnormal serum FLCs can determine the risk of transformation to overt multiple myeloma. These parameters are used to help determine the frequency of monitoring.
What are the management options in myeloma?
Symptomatic myeloma
Symptomatic myeloma usually requires immediate treatment (Figure 3). For those younger than 65 years of age, or those aged 65–70 with few comorbidities, autologous stem cell transplantation is the standard of care. Patients typically receive 3–6 cycles of induction treatment, aiming to achieve a complete or near complete response, prior to receiving their transplant. There have been significant advances in the choice of induction agents and several new agents that have improved responses in multiple myeloma are now available. The best results appear to be achieved with a combination of steroids, cytotoxic chemotherapy (such as cyclophosphamide or doxorubicin), and a novel immunomodulatory agent (eg. thalidomide or lenalidomide) or proteasome inhibitor (eg. bortezomib).12
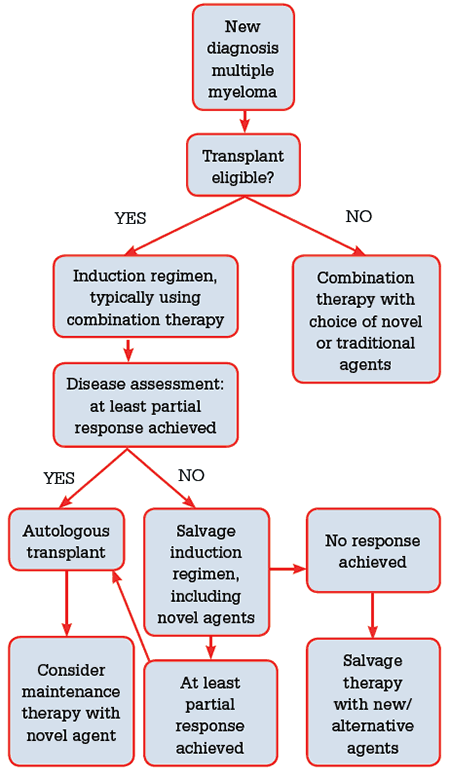
Figure 3. Treatment flow chart for newly diagnosed multiple myeloma
The choice of novel agent is often determined by comorbidities and side effect profile; bortezomib is preferred in the setting of renal impairment or advanced disease, while lenalidomide is preferred in the setting of peripheral neuropathy. Following transplant, patients are often offered maintenance thalidomide, with or without prednisone, for approximately 12 months. Despite a clear improvement in progression-free survival, this strategy may be poorly tolerated due to medication side effects and adversely impact on patient quality of life.13 Lenalidomide maintenance also appears to prolong progression-free and event-free survival14 at a cost of increased toxicity and second malignancies,15 but is not subsidised under the Pharmaceutical Benefits Scheme (PBS) unless patients are intolerant to, or have disease progression on, thalidomide.
Those ineligible for transplant may be treated with a combination of steroids, a cytotoxic agent (such as cyclophosphamide) and thalidomide or bortezomib. The role of maintenance therapy following induction treatment remains unclear in this group.12
Bisphosphonates are a critical adjunctive therapy employed in symptomatic myeloma, and offer a clear reduction in pathological vertebral fractures, pain and other skeletal events. Intravenous monthly zoledronic acid has also been reported to improve overall survival.16 Potential side effects include hypocalcemia, renal dysfunction and osteonecrosis of the jaw (ONJ), which may present with localised pain, swelling or exposed bone. Patients should have all necessary dental work completed prior to commencing bisphosphonates to minimise the risk of ONJ.
Smouldering myeloma and MGUS
Treatment for those with MGUS and smouldering myeloma consists of watchful waiting, with serial monitoring of the paraprotein and frequent assessment for the development of myeloma-related organ or tissue impairment. Australian guidelines suggest monitoring of MGUS occur 3–12 monthly, depending on the individual patient risk.12
Case study
Matt, 44 years of age, presented to his general practitioner with a two-week history of sudden onset lower back pain. X-ray revealed a thoracic vertebral crush fracture, and he was prescribed analgesia. He was previously well with no significant medical history, including no history of corticosteroid use, and denied a history of trauma. He presented to the emergency department four weeks later with ongoing back pain, and was prescribed additional analgesia and discharged. He re-presented to the emergency department within a week due to uncontrolled pain, and blood tests were taken for the first time. These revealed anaemia with a haemoglobin of 92 g/L (135–180), a corrected calcium of 3.17 mmol/L (2.1–2.55), a creatinine of 256 µmol/L (60–110) and an eGFR of 24 ml/min/1.73 m2. PTH was appropriately suppressed at <0.5 pmol/L (1.6–7.2). In addition, he had a raised total protein of 122 g/L (60–80) with globulins of 88 g/L (10–42). A serum electrophoresis was subsequently requested, showing an IgAK monoclonal band, and bone marrow biopsy confirmed a diagnosis of myeloma.
Conclusion
Multiple myeloma can present a difficult diagnostic issue, as there are a wide variety of presenting symptoms. Suspicion for myeloma should be raised when a patient presents with a constellation of unexplained symptoms, such as an ‘osteoporotic’ crush fracture or bony pain in a young person, hypercalcaemia with a normal PTH, anaemia with normal haematinics, and/or renal impairment without a history of diabetes or autoimmune disease. Establishing the diagnosis rests on identifying a monoclonal paraprotein in the serum and confirming the presence of myeloma-related organ or tissue injury, followed by haematology referral to establish evidence of bone marrow plasmacytosis, and for future management. With the introduction of novel immunomodulatory agents and proteasome inhibitors, newly diagnosed patients are enjoying a longer life expectancy than their historical peers. However, the role of the general practitioner is still crucial in helping to make an early diagnosis, allowing patients to be identified and treated without potentially harmful delays.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.