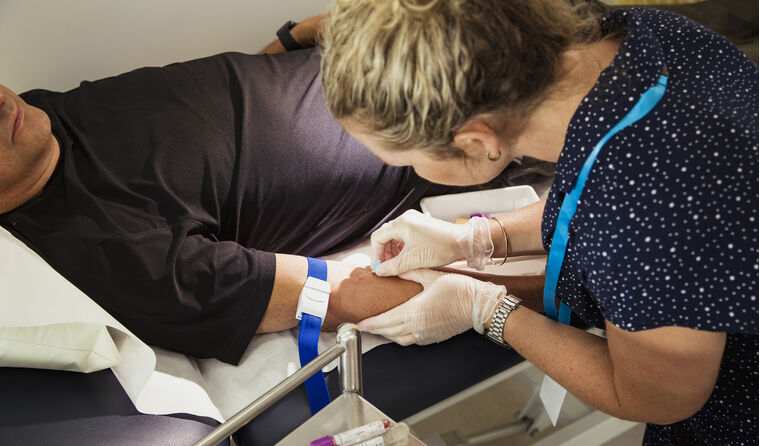
Among the 1 November changes introduced to the MBS, is the new item 73812 for glycated haemoglobin (HbA1c) point-of-care testing (PoCT).
In addition to tests performed through a laboratory, the service can now be provided by, or on behalf of, a medical practitioner in a general practice, with a fee of $11.80 covering the PoCT.
To be able to claim the PoCT item, practices need to be accredited under the Australian Commission on Safety and Quality in Healthcare’s National General Practice Accreditation Scheme against both the RACGP Standards for general practices and Standards for point-of-care testing.
The RACGP developed the Standards for point-of-care testing in 2018 to support the implementation of PoCT in a general practice setting, proposing that with appropriate training, technical support and quality assurance, PoCT in general practice is as clinically effective and safe as laboratory pathology testing.
The college has been actively advocating for PoCT item numbers for general practice to support quality care, while also recognising the value in providing enhanced and timely clinical management for chronic disease.
‘PoCT for HbA1c will give GPs the ability to make immediate and informed decisions regarding the care of their diabetic patients, improving clinical management,’ Dr Louise Acland, Chair of the RACGP Expert Committee – Standards for General Practices, said.
‘PoCT is patient-centred − it is convenient, it provides opportunities for increased patient engagement with the practice team, and has the potential to improve health outcomes for our patients.’
Dr Gary Deed, RACGP Specific Interests Diabetes Chair, agrees the new PoCT item number further validates the importance of primary care in managing people with diabetes.
‘It [the item number] will allow a seamless in-office assessment of those patients who have not been able to obtain one of the important and necessary glucose measures, HbA1c, that we use to help manage diabetes therapy, advice, and also for complication prevention,’ he said.
‘It will complement the clinical assessment measures that are needed, for example blood pressure, cardiovascular and feet examination.
‘It also supports those patients who struggle to have regular follow-up and thus those who may carry higher risks related to their diabetes.’
According to Dr Deed, the item number provides more choice to both practices and patients when it comes to HbA1c testing as PoCT becomes more accessible, which would particularly benefit regional and rural settings.
In 2020, the Department of Health proposed to include the RACGP Standards for point-of-care testing as an addendum to the National Pathology Accreditation Advisory Council (NPAAC) requirements, within which general practices will be referred to.
The RACGP provided feedback to numerous consultations regarding the NPAAC requirements for PoCT, which are appropriate for laboratories but not for general practice, stating that Medicare rebates should be available and at least equivalent to those currently available to pathology providers.
Dr Deed said the new MBS item supports this alignment.
‘The technology used in PoCT has been validated against standards, so GPs should be confident in the results they obtain from PoCT,’ he said.
In addition to practices being required to meet the RACGP Standards before implementing the PoCT technology, Dr Deed said there are other criteria specific to diabetes care that GPs should be aware of.
‘The MBS rebate is only for monitoring of HbA1c with appropriate intervals of no more than four per year, and it is not indicated as a diagnostic test,’ he said.
‘The investigations necessary for wholistic diabetes care also encompass broader investigations including lipid profiles and urine protein assessment, and these may still require timely pathology assessment.
‘The equipment is an investment cost for practices and owners need to assess the costs of consumables, the need for accreditation versus the provision of care benefits.’
Dr Acland said general practices should also consider the accreditation timeframes.
‘There is a one-off 12-month registration period for PoCT assessment,’ she said.
‘After that, the assessment cycle is three years, in line with practice accreditation. Assessment for PoCT is an onsite assessment. Accreditation for PoCT is only required for access to MBS payments for HbA1c.’
The service can only be claimed a maximum of three times per patient in a 12-month period. It may not be claimed by a patient if a total of four other HbA1c testing items − laboratory or PoCT – have already been provided to the patient in the last 12 months.
An equivalent item (73826) was also introduced to the MBS on 1 November for nurse practitioners working in an eligible general practice.
More information on the legislation for the new item for medical practitioners is available on the Federal Register of Legislation’s web page.